Gardening in Space
By David Jones
On the space shuttle Columbia, an experiment explores chemical growth without gravity and the challenges of orbital science
On the space shuttle Columbia, an experiment explores chemical growth without gravity and the challenges of orbital science

DOI: 10.1511/2002.33.454
On March 6, 1993, scientists around the world awaited the launch of the space shuttle Columbia for the D2 research mission. Its dozens of experimental packages were mostly German, but my little British chemical garden took its place among those from other countries.
The seven astronauts were strapped in place, and the launch computer counted down through the endless checks...and aborted the launch with three seconds to go. For hours, the astronauts lay over a tank of liquid hydrogen before the long abort procedure allowed them to be rescued. The pending scientific experiments would wait even longer.
The aborted launch disappointed most everyone involved. The D2 mission was a joint venture between NASA and the German space agency DLR. Years before, the two agencies determined all of the big experiments, but my little one fitted in a bit of spare space and free time. While others lamented the aborted launch, it gave me a tiny ray of hope, because my chemical-garden experiment was in trouble. One of its two units was leaking. The aborted shuttle would remain upright on its launch pad for at least a month before NASA would try to launch it again. In that time, many experiments would have to be repacked. The biological ones, for example, would not keep. The repacking crews would come close to my chemical garden in a few weeks. They could exchange the leaky unit for a spare, if I could get one ready and out to Cape Kennedy in time!

Courtesy of David E. H. Jones.
To explain how I had got myself in this fix, let me tell a story from 1982. George Pimentel was showing me the chemistry department at the University of California at Berkeley. The workshop there was like the one I knew in the chemistry department at the University of Newcastle, but about 10 times the size. "Here," said Pimentel, "is where we built the spectrometer that went to Mars." Wow! I thought. What a wonderful boast. So, when I had the chance to do some space science of my own, I leapt at it.
My chance for space science came in 1988, when I got talking with Ulrich Walter, a German astronaut in training for the D2 shuttle mission scheduled to be launched in 1991. It still had room for a few "pocket experiments" that could be tried out in spare moments. He agreed to put my ideas to the D2 scientific committee.
As a chemist, I began wondering about space chemistry, in particular the "chemical garden." In 1648, the German chemist Johann Rudolf Glauber first described a chemical garden in his book, Furni Novi Philosophici, which translates as the "New Scientific Furnaces." Many of us grew these gardens as schoolchildren, by dropping crystals of various metal salts, such as cobalt sulfate, into water-glass, which is a solution of sodium silicate. Frond-like tubes?composed of insoluble metal silicate?grow upward from the crystals over minutes or hours. The reacted solution is less dense than the water-glass. It streams upward and entrains the fronds, which grow upward. Deprived of an "up" direction in which to grow, what would a chemical garden do? Would tubes form at all? If not, how would the reaction behave? I could only make bad guesses, so it seemed a good experiment.

Courtesy of David E. H. Jones.
By March 1992, with the launch already greatly delayed, Walter told me that the space agencies accepted my proposal. I had 9 x 9 x 16 centimeters of space, which was later renegotiated to 13 x 16 x 17 centimeters, 5 kilograms of weight and an hour of still- and video-camera time. I also received a delivery deadline of September 1, 1992, less than six months away. The project seemed deceptively easy: Just squeeze as much chemistry as possible into the space. Claus Kramp, the engineer at DLR Cologne, Germany, in charge of all experiments on the D2 mission, would keep an eye on my progress. The experiment would be the "Microgravity Chemical Garden," where microgravity is NASA-speak for almost no gravity.
Trouble started at once. An experiment that takes a few moments in a beaker in the laboratory turns amazingly complicated in a spacecraft. Safety raised one problem. NASA sets very high safety standards for things that get into a shuttle. For instance, my apparatus needed at least two layers of containment, each to pass rigorous leak and pressure tests, with something between the layers that could provide absorption?in case of a leak. Timing created another potential problem. Could the reaction, slowed by the absence of gravity, get anywhere in an hour? Even worse, the apparatus must remain stable during months of waiting, and yet must operate at once when called upon. To meet space-agency deadlines, I needed to settle quickly on a design.
In April 1992, I gave the job to the mechanical engineering workshop of Newcastle's chemistry department and sent off a preliminary drawing to DLR Cologne. As with all designs, this one powerfully stimulated my creativity. The ultimate apparatus looked nothing like the preliminary design. I gradually refined my ideas. NASA would provide the cameras. I would provide the apparatus in a stainless-steel box. It would contain primary and back-up chemical-garden units and mountings for the cameras. Furthermore?a crucial decision, as it turned out?the engineers and I would make three units. I wanted to work one on the ground in parallel with the two in space.
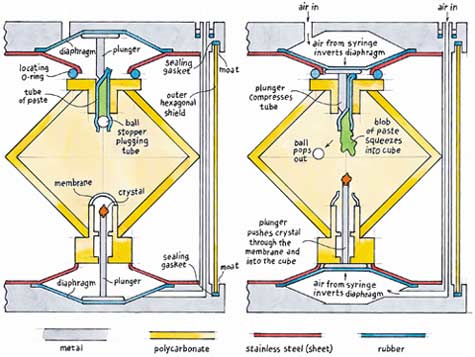
The Newcastle chemistry workshop grew fully involved in this project. Roland Graham and Bruce Atkinson, in particular, committed themselves to it. The central problem was how to inject a metal-salt crystal into a silicate solution. I devised two mechanisms, and we used both of them. One pushed a crystal on a rod into the solution through a thin polyester membrane that wiped away any air from the crystal. This hemispherical membrane would also deform to take up any volume changes with temperature. Better still, it could deform to permit an input from the other injector. The other injector added a metal salt as a saturated solution, thickened to a paste. The paste would be retained inside a silicone-rubber tube and sealed by a Teflon ball. An advancing rod?in this case a knitting-needle tip?would compress the tube, and the ball would pop out. The paste would be extruded into the solution like toothpaste and would grow a chemical garden.
Tom Dunne
While I struggled to express these ideas in hardware, the other team members tackled the units themselves. Each unit would contain the sodium-silicate solution inside a cube that was 4 centimeters on a side. No outside contractor could make these cubes, because NASA wanted them in polycarbonate, as used in bullet-proof glass. Luckily, I came across a wonderful glue for polycarbonate. Although very difficult to handle, it made excellent joints. We made a cube by gluing together six mitered faces. Their edges were reinforced with a polyester strip. In the completed device, a cube would sit as if balanced on one corner.
Each cube included an injector of each type. The crystal injector pushed its crystal up from the bottom corner of a cube, and the paste injector pushed its charge down from the top. A camera on a mount could swing round a whole unit and look into three cube faces, in a true x-y-z scrutiny. For added protection, the whole thing was placed in a closed hexagonal polycarbonate shell, which was designed to withstand 1.5 atmospheres. Even if the shuttle lost all of its air, the excess pressure in each sealed garden would only be 1 atmosphere. And the astronauts would have other worries!
Each unit had a light-alloy end-face on the top and bottom. Each end-face had a depression to take a rubber diaphragm. The diaphragm, when inverted by air supplied from a syringe, drove its injector. To retain the inverted diaphragm, each end-face bore a shallow lipped cone. It also bore a milled hexagonal "moat" with an internal rubber sealing gasket. The two end-face moats compressed the hexagonal outer shell between them. Six threaded rods clamped the end-faces together, and one was hollow. Air for the bottom diaphragm was to be injected from the top end-face through this rod. The astronaut would mount the unit in its photographic frame, both stuck to the shuttle bench by double-sided tape. He would drive both diaphragms from the top face with the syringe. With the camera he would record how the gardens grew.
Roland had two inspirations. As retainers for the diaphragms, he pressed six shallow cones from stainless steel sheet?much faster than carving them from the solid as I had suggested. He also built a neat little bending machine to form each hexagonal polycarbonate-sheet shell, which was to be closed by my splendid glue. Those end-faces with their moats and depressions gave Bruce lots of milling problems.
With the units designed, the major remaining problem was how to mount the cubes inside them. I made a cardboard mock-up, brilliantly copied in stainless steel sheet and mesh by Roland. Each mount fitted on the bottom end-face, and took a cube on top. Space inside each mount held absorption material to mop up spilt solution if anything went wrong. The crystal injector went up through its middle. On the opposite end-face at the top, the paste injector came down.
All of this was slow, inventive effort. We wasted months going up blind alleys. Even the crucial components, the injectors themselves, were never properly tested. By August 1992, it was embarrassingly clear that we would miss the delivery date of September 1. Fortunately, the launch date for the D2 mission was slipping as well, but not fast enough. Furthermore, DLR started getting suspicious of my stories of our progress, and so did NASA. Both disliked the way the design kept drifting long after it should have been frozen. Finally, on September 16, Kramp phoned me with bad news, but a glimmer of hope. The project had missed its deadline and was formally cancelled. Nonetheless, if I provided a new safety document by September 30 and the hardware itself by October 16, it might still be accepted.
I agonized over Kramp's offer for a day or so, but then acknowledged defeat. I was ragged with fatigue. I couldn't see how to go any faster, or how to complete the apparatus without further refinements to the design. I offered to cancel the whole project. To my surprise, Kramp refused the cancellation. DLR liked the experiment, and?unofficially?the launch date kept slipping and many other experiments were behind as well. According to Kramp, even a mid-November delivery date might be acceptable. I began to realize that he had been fighting hard on my behalf. NASA would not allow anything on board that was not certified to meet its specifications. If the shuttle fell out of orbit because of some fault in the Microgravity Chemical Garden, Kramp would face the blame, not I. He had to convince the safety officials at both DLR and NASA that we were developing a safe experiment. The shifting and imprecise details that I kept sending him were undermining his position.
I agreed that mid-November was a reasonable date. Nevertheless, eight months is a record for developing any space experiment! Most of the development money was already spent, so DLR lost little by letting our project run till mid-November and seeing what we could create. Roland and Bruce felt that, with a bit of overtime, we should meet the deadline. At that point, they were constructing the photographic mounts and the flanges for the video and the still cameras, where Bruce made a neat improvement to my design: a sliding adjustment to help with centering and focusing.
The next hurdle was a vibration test. A rocket burns in turbulent flow, so its roar and vibration shakes everything on a spacecraft with frightening violence. The European Space Agency at Noordwijk, Holland, runs a broad-band vibration rig, which would be free on November 9 and 10.

Courtesy of David E. H. Jones.
We began a mad rush to complete the apparatus to make those dates. I'd never designed anything to pass a vibration test and approached it entirely from first principles. I decided to bed the units on soft foam rubber, so that they could stay fairly still while the box shook round them. John Meehan, my Noordwijk contact, helpfully sent me a large block of "space grade" foam?designed not to crumble or outgas unacceptably in a spacecraft environment. An evening of careful sponge artistry with jigs and a lashed-up hot-wire cutter gave me the various sheets and spacers I needed to hold the units in the box?another splendid piece of Roland's sheet-metalwork.
One main thing still concerned me. At the bottom, a single nut alone held each cube down on its stainless-steel mounting. At the top?the paste-injector end?nothing supported a cube. Heavy with silicate solution, it would be shaken around on that mount and could easily work loose. How could we hold it down from the top end? If the top end-face steadied a cube, it could not also hold down the moat gaskets, and they would leak.
We worried about this for a fortnight, while designing and perfecting the rest of the hardware, such as improved paste injectors and the pipes, fittings and internal ducts that would carry air to the actuating diaphragms. Finally, a solution came in a way that, for me at least, was psychologically unique. I never believed in joint creativity. Individuals have ideas, not groups or committees. Yet, I do not know which of us proposed the ideal way of locating and damping the cube at its paste-injector end. The idea came at the end of many converging suggestions?a true collective invention. We saw that a simple rubber O-ring, mounted on the lip of the cone of the top end-face, could bear on the cube inside and retain it resiliently. We merely machined the top face of the cube flat, so that the ring would sit stably wherever it found itself and could deform freely. The ring would float on and retain the cube elastically, and it would still allow the hexagonal outer shell to load its gaskets.

Courtesy of David E. H. Jones.
With the last design decision made, everything could be machined to final size. Roland and Bruce set up a sort of assembly line on which each cube was matched to its mount, and each outer shield was machined to matching length, because each of the three hand-built units was slightly different. We rushed desperately to get two of them finished and filled in time for the vibration test, and we managed it by November 9. With an anxious heart I rose at 5 a.m. the next day to catch the flight to Holland and face the first serious outside test of our efforts.
The French engineers at Noordwijk were contemptuous of the little box that they strapped on their huge computer-driven shaking machine. It took them at least an hour to align it dead square and bolt it down firmly. Three times they did this: with the box flat, then on its longer edge and, finally, on its shorter edge. The roaring vibrational ordeals took only two minutes each. At the end of it all, I opened the box with some trepidation, prepared to shake out the pieces, but nothing seemed damaged. Meehan checked the parts for out-gassing potential, but there were no problems. The Microgravity Chemical Garden passed its vibration test!
The last tests were scheduled for November 24 and 25. A DLR inspector would come to Newcastle to see the final package assembled and tested and take it back to Cologne. In preliminary tests, we loaded an outer hexagon to 2 atmospheres?half an atmosphere more than it needed to sustain?and it didn't leak. We also pressured a spare cube to destruction. To enliven this event, we held a workshop wager on the outcome. I guessed that the cube would fail at 6 atmospheres, general workshop opinion favored 8 and Roland reckoned 10. In fact, it failed at 12 atmospheres?amazingly good. The night before the inspector arrived, I found and sealed another small leak, by cementing each silicone-rubber diaphragm to its machined rim "gutter." Mercifully, it worked, and the units passed their final series of pressure and filling checks. The last job of all was to insert the sealing transit screws that prevented the injectors being affected by changes of pressure during air transport.
Then the wait began. The launch, by then planned for February 18, 1993, was later rescheduled for March 13. On December 10, 1992, the acceptance inspectors at Cape Kennedy discovered a small chemical garden growing from the paste injector of the primary unit. I began to worry. By February 1993, I thought I realized what must be happening. The silicone-rubber tube containing the paste was acting as an osmotic absorber and was gaining pressure. I soon verified this idea in the lab. The paste injectors in both units would inevitably leak. The longer launch was delayed, the worse everything would get.
Courtesy of David E. H. Jones.
The aborted launch of March 6, 1993, gave me another bite at the cherry. I blessed my early casual decision to build a third unit. I got the spare ready, and replaced its silicone-rubber tube with butyl rubber, which is far less osmotic. The best I could find in the time was bicycle-valve tubing from a local cycle shop. Still, time was short. On April 7, a DLR inspector arrived in Newcastle to oversee the pressure and acceptance tests of the third unit. In the tearing hurry to prepare and fill it with magnesium chloride paste and nickel sulfate crystal, a small contamination occurred. A fiber from my woolly pullover stuck to the sealing grease of the paste injector and went into the cell with it. By the time I noticed, there was no time to take everything apart again. The wool fiber would just have to be part of the experiment.
The DLR inspector took the third unit to Cologne, from where, providentially, Walter was shortly flying back to the United States. He had visited Germany for last-minute training and discussions. The unit went with him and reached the shuttle just in time. The repackers swapped it for the leaky unit and sent the dud back to me.
When I examined it, I was horrified to find that both injectors were leaking! Later, Walter told me that NASA technicians routinely pumped up the shuttle a bit to see if it lost air. My brilliant scheme for driving the injectors with air pressure from a syringe had just been asking for trouble. Awaiting relaunch, however, it would not be pumped up again. Ironically, I had already provided the perfect counter to this problem?the sealing transit screws in each unit, to guard against the pressure changes of air transport. Even more ironically, I had asked that these should be removed once the apparatus was safely in the spacecraft.
Desperately, I phoned the local meteorological service. What was the air pressure and pressure forecast for Florida? They told me that high pressure was extremely rare in the equatorial zone, although it could drop sharply if a cyclone passed over. If the shuttle took off as hoped, and its internal air pressure remained steady, the chemical garden might still work.
The D2 mission finally took off on April 26, 1993. In a mood of mixed hope and despair, I arranged to go to the control center near Munich. Despite my worries about atmospheric pressure, I wanted to be present when the chemical-garden experiment was carried out. I knew this experiment better than anyone else, and a word from me might be vital. I brought along the refurbished third unit to operate in parallel with the ones in orbit. That at least ought to work.
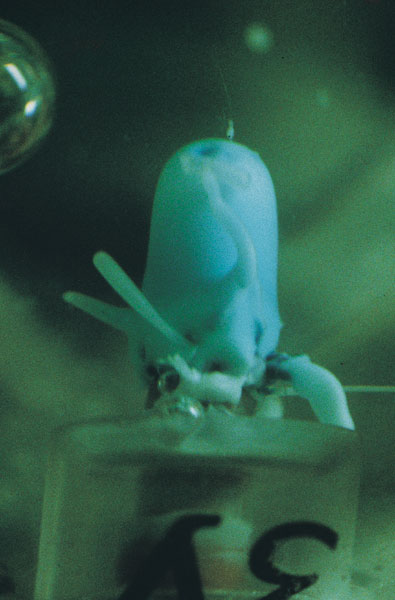
During the actual experiment, though, I wasn't needed. Walter worked both injectors on both units, and gardens grew from two of them. The first chemical garden my apparatus grew in space was expelled from a piece of bicycle-valve tubing compressed by a knitting-needle tip. Walter cleverly arranged for it to be imaged by still and video cameras looking simultaneously through two faces of the unit?something I hadn't considered. Later, when he worked the other unit, he took pictures with the still camera only. I couldn't guess what might have happened to the other injectors, but two gardens out of four seemed ample success. I duly operated the third unit successfully, as something to do. On the ground, both injectors worked.
The D2 mission went through its multiplicity of tasks, stayed an extra day in space and landed safely and triumphantly on May 6, 1993. Walter told me that he had also taken photographs of the garden experiments after a further 48 hours of development. Months later, I learned that the video looked good, the film was being developed and the garden apparatus had been safely unloaded and returned to DLR Cologne. The gardens themselves had survived re-entry and transport back to Cologne largely undamaged.

Courtesy of David E. H. Jones.
I was amazed. I expected to have film and video to interpret, but I assumed that the gardens themselves would be destroyed by the buffeting of re-entry. Yet hardened by the long-completed reaction, and with liquid cushioning around them, the space-grown gardens themselves survive re-entry, an air journey to Cologne and later one to my Newcastle laboratory. But there was a snag. Never having expected this, I had not designed the injectors to be retracted or removed. In any case, exposed chemical-garden structures are unstable in the air.
It took months to solve these unexpected problems. To gather as much evidence as possible, I took photographs of the two gardens in their cells from all angles. They were quite unlike anything I'd grown on Earth. A cobalt sulfate crystal created a garden with many fine tubes, seemingly tangled and convolved near the crystal, but extending dead straight across the cell when they grew away from it. Some even bounced off the cell walls and carried on growing. A magnesium chloride paste, with a little nickel chloride as a compositional marker, grew a bulbous garden with thick shortish fingers. That unit included the accidental textile fiber, but the garden grew right through it as if it wasn't there. Indeed, the video showed how soft and fluid this garden had been in the growing. After a number of trial runs, I managed to extract the space-grown chemical gardens from their cells and mount them for further examination without damaging them too much.
One outcome was quite unexpected?and very welcome. I found a third space garden. The calcium chloride paste injector?opposite from the cobalt sulfate crystal?made a seemingly flocculent precipitate of calcium silicate around a hollow cylinder of the stuff. The emptied cell, however, included a thin, coherent coating on its faces, with thicker deposits at the edges and in some corners. This is how an immiscible wetting liquid spreads itself in a cubic cell in microgravity. It can't mix with the solvent, so it displaces the solvent from the walls and corners of the cell and spreads as a thin film. It is the ultimate in surface growth.
I returned to the cobalt and magnesium gardens after finding a way of stabilizing them for the scanning electron microscopes at Newcastle's materials science department. I took hundreds of pictures, and it took me years to make sense of the results. The liquidness of the growing garden was the thing that seemed to matter. The calcium garden grew as a pure liquid, wetting the walls of its cell. The magnesium garden grew largely as a liquid, engulfing the textile fiber in its path. That garden also expanded like a liquid around the injector, where it happened to grow along it. It also formed numerous "fingers" that were formed in a fluid and inflatable way, to judge from the video of their growth. Even the cobalt garden grew a big fluid "finger" alongside its many tubes, though those tubes must have grown in the same solid style which that garden shows on Earth.
So that was the story. A chemical-garden reaction doesn't need gravity. Without the rapid supply of new reagents by gravity, a space chemical garden still grows tubes like an Earthbound one, but more slowly and perfectly. In microgravity, the reaction initially forms a liquid, which can stay fluid for a long time. Indeed, the reaction can grow thick fingers in a novel manner, by continuous plastic flow. The shape thus formed slowly hardens. The final result is a rarity in physical chemistry?a new example of a fluid instability. Later, I found a piece of physics which fitted all my results. The Laplacian fluid instability, described in most detail by Martin E. Glicksman and his colleagues for the solidification of melted succinonitrile, fits the fluid formation of chemical gardens in microgravity.
It seems a vast amount of thought and effort to reach such a simple conclusion. But of course it isn't a conclusion. No research reaches a conclusion. Instead, this project started many trains of thought, most of which are still running. But thanks to that chance remark by George Pimentel at Berkeley, I can take you to the workshop in the chemistry department at Newcastle and say, "This is where we built the chemical garden that went into orbit!"
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.