Cats and Rats
By Roald Hoffmann
On catnip, clover and a chemical clue to Stalin's death
On catnip, clover and a chemical clue to Stalin's death

DOI: 10.1511/2003.26.308
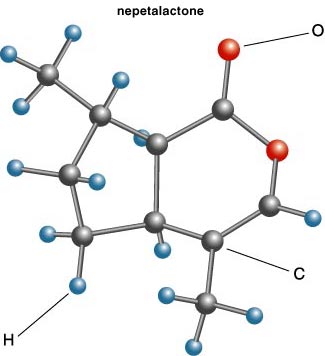
Cats are crazy about catnip. So every pet shop does a brisk trade in run-of-the-mill catnip toys, and some over-the-top catnip articles, such as those shown in Figure 1, are offered on the Web. The active principle in catnip is called nepetalactone, which is a so-called cyclopentanoid monoterpene—a pretty simple organic compound. My colleague, Jerry Meinwald, determined its structure (shown at right) about 50 years ago. Nepetalactone, synthetic or natural, induces in most cats a psychosexual response of stretching, rubbing and curling. The behavior is elicited at concentrations as low or lower than one part per billion. And not only in domestic cats, but also other Felidae members, including lions. This I would like to see.

Image courtesy of www.catnippizza.com
But what's in it for the catnip plant, a member of the mint family? Surely Nepeta cataria did not evolve its signature compound to intoxicate cats. It does not need them for pollination or propagation. Might another creature be the intended target for nepetalactone? Tom Eisner asked this question almost 40 years ago. In a paper so clear and free of jargon (typical of Eisner's work; see Macroscope) that I can read every word of it with my first-year chemistry class, he describes his findings. Here is a piece of it:
This possibility [that catnip may be a defensive substance, protecting the plant against phytophagous insects] was investigated by a series of simple experiments.
One of these consisted in observing the response of a variety of insects to the vapors emanating from the tip of a fine capillary tube filled with pure liquid nepetalactone, and pointed to their bodies from a few millimeters away. The insects tested (Table 1) were a mixed assortment that had come to rest at night on an illuminated surface. The majority (part A) showed a distinct avoidance response, which varied somewhat with the particular species. The caddis-flies flew away. The alleculid beetles fell to the ground (as do many beetles when disturbed). The remainder simply turned away from the capillary and walked off.
So nepetalactone is an insect repellant. This might be of evolutionary advantage, part of a web of defense that the catnip plant uses to protect itself from herbivorous predators. As my friend Haruko Kazama at the International Christian University in Tokyo says, "Plants are sessile not because they are primitive and cannot move, but because they have such exquisite mechanisms to sense and respond to their environment. They do not need to run away...."
Emma Skurnick
Remarkably, nepetalactone next turned up in an insect, the walking stick. Both life forms are trying to avoid being eaten, and of course they are not alone in this desire. Several plants and animals use other, similar molecules as insect repellants. Nepetalactone is reported to be stronger (by weight) than DEET as a mosquito repellant. And, in a bizarre twist of its usual role, the compound is made (along with related molecules) by female aphids to attract males. Unfortunately, novel use can be risky: Parasitic wasps that prey on the aphids can also sense the molecule. This has led to commercial production of nepetalactone to control aphid infestations via wasp recruitment.
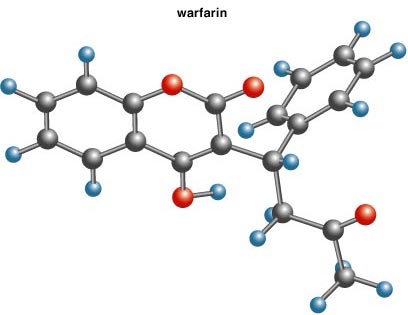
This part of the story begins (for me) in St. Vincent's Hospital in New York City, where my mother is given Coumadin after a heart attack. A few days later, my eye is caught by a front-page article in the New York Times reporting a recent study from the New England Journal of Medicine. In it, Paul Radker and his colleagues at the Brigham and Women's Hospital in Boston show the effectiveness of a new, mild regime of Coumadin in preventing blood clots. Because of my mother, I read this with more than usual interest, and note the generic name of the drug, warfarin.
Emma Skurnick
Something bothers me about the name. Who would give a life-saving anticoagulant a moniker out of Mordor? A little research reveals that warfarin, whose structure is shown above, first found use as an effective rat poison! That explains its name, I think; it has also been marketed as Marevan, Dethnel, Rodafarin and Frass-ratron—equally fitting names.
But . . . while my intuition seemed to lead me in the right direction, I was wrong about the origin of the name. It turns out that the name warfarin comes from the initials of the Wisconsin Alumni Research Foundation, which patented it! And from coumarin, a natural product of which it is a derivative.
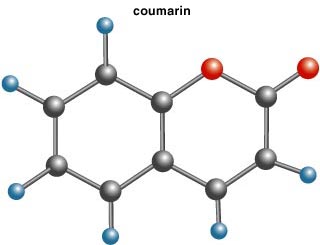
The story of the anticoagulant-cum–rat poison begins with an agricultural strategy of the early 1900s, the introduction of sweet clovers in the North American prairies. In 1924 ranchers in Alberta, Canada, observed that cattle feeding on moldy sweet clover were dying from a hemorrhagic disorder; the active agent was found to be dihydroxycoumarin (coumarin itself is shown below). This molecule was synthesized within a year and quickly found use as an anticoagulant. Meanwhile warfarin, the derivative of coumarin, was developed by University of Wisconsin biochemists Mark A. Stahmann and Karl P. Link, and used as a rodenticide. It took 10 years for it to enter clinical practice as a blood-thinner.
Emma Skurnick
Coumarin smells good—the parent molecule is described in the Merck Index as having a "pleasant, fragrant odor, resembling that of vanilla beans...." There is even a story of it being used to enhance vanilla, a practice banned, for obvious reasons, some time ago. Nevertheless, some tobacco-leaf preparations were soaked or "cased" in coumarin to add aroma.
So what's in it for the clover? Defense, again. And sweet clovers aren't the only greens using coumarins. Many plants make use of furocoumarins, in which a coumarin is bonded to a furan ring, as protective substances. May Berenbaum of the University of Illinois has shown that such compounds are quite effective in protecting parsnips from insect predators (except for the ones that have evolved countermeasures). A further quirk is that furocoumarins are photodynamic—their toxic effects in the animal are triggered by sunlight. Humans are not immune: There's a report in the literature on "celery phytophotodermatitis in a chef." And an unnamed young woman made the medical literature with a case of "Club Med photodermatitis" following a poolside game of rolling limes between her thighs. Watch out for veggies.
The book Stalin's Last Crime: The Plot Against the Jewish Doctors, 1948–1953 (HarperCollins) has just been published by Jonathan Brent and Vladimir Naumov. Relying on a secret account of Stalin's last days in March 1953, it suggests that he might have been poisoned by . . . warfarin. Easy to make, the compound seems to have been available at the time, and Stalin's symptoms—brain hemorrhage and stomach bleeding (the latter excised from the published coroner's report)—fit. The Brent and Naumov hypothesis needs to be confirmed by further analysis, but the book, which also covers other topics implied by the subtitle, has no trouble adducing reasons (or conspirators) for poisoning the dictator.
The molecules in these stories are simple ones. Plants make coumarin from an amino acid, phenylalanine. Nepetalactone, like other monoterpenes, begins from two five-carbon chains, in turn assembled from two-carbon building blocks. The O-C=O, or lactone, unit in warfarin and nepetalactone rings is not even deep in their ancestry; it's just an ending—an ordinary, common way for organic acids and alcohols (or aldehydes) to react. Lactone rings are found in many natural products; there is a huge one in the antibiotic erythromycin.
Vignettes like these are just a part of the life-enhancing chemical games in the natural world. As Tom Eisner and Jerry Meinwald have shown, insects are magnificent chemists. But all species use both simple and complex chemistry to execute the inspired tinkering of evolution. It's downright fascinating to learn how animals and plants do it. This is biosynthesis, a rich nexus of chemistry and biology. The diversity of life mediated by chemistry—in mint and clover, giving pleasure to cats, scaring away insects, causing bleeding in cattle, fragrant to us, preventing clot formation—makes a chemist feel good.
And not just a chemist.
© Roald Hoffmann
Thanks to the students of Chemistry 106 for leading me to learn about nepetalactone and warfarin, to Haruko Kazama and Jerry Meinwald for their comments, and to Dean Tantillo for the molecular structures (each only one of several stereoisomers), drawn with Ball & Stick, version 3.7.6, by Norbert Müller and Alexander Falk, Johannes Kepler University Linz, Austria.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.