
This Article From Issue
March-April 2010
Volume 98, Number 2
Page 117
DOI: 10.1511/2010.83.117
I meet Ansgar Bach first through his writing. He sends me an endearing account, in German (which I can read, because I was once a refugee in post-World War II Germany), of a trip he took to New York. In the middle of the 47th Street Diamond District, Ansgar spots an oasis (sadly gone today): The Gotham Bookmart. In it he finds my second poetry collection, Gaps and Verges, signed. The bookstore sign says “Wise men fish here.” Ansgar fishes, writes about it, sends me what he writes and catches a new friend.

Books mean much to Ansgar. I discover that when I do what can only be done today, an Internet search on his name. From the many sons of Bach and still more Bach festivals, I excavate an interesting fact: He is an unusual chemist. For although he does at this time have an institutional affiliation—the Department of Chemistry at the Free University of Berlin—he also runs a small business: Literarisch Reisen. The phrase is resonant; in one sense it means “Travel in Literary Fashion.” And indeed Bach’s firm organizes excursions in his region of Germany that follow the footsteps of Thomas Mann or visit the sites of a series of stories by E.T.A. Hoffmann or Heinrich Heine. Very German. Very literary. And most unlike what chemists do.
Ansgar is on a path quite different from that of most academic scientists. Like him, like many other scientists, I have wide-ranging interests that include literature and art. But can one professionally embrace those multiple interests? Can one successfully pursue scientific research only on a part-time basis, with the rest of one’s attention focused elsewhere, no matter how gripping or worthwhile the other pursuits might be?
Crossovers
Ansgar and I continue to correspond. He sends me a detective story he has published, Ukrainische Verbindung (The Ukrainian Connection). In German such stories are called Krimis, for Kriminalromane. The story, quite an exciting one, begins in a German paint factory, and part of the action takes place in the Ukrainian city of L’viv. Another connection, for I was born in Zloczów, some 60 kilometers from L’viv! My father had gone to the Lwów Polytechnic University, as it was called in Polish days. Earlier, during the Austro-Hungarian days, L’viv was Lemberg. A crossroads of the world it was, our historic region of Galicia. And it was also a place for waves of ethnic cleansing.
One day in 2002 Ansgar comes to visit. He is spending a month in a lab at the State University of New York at Buffalo, practically next door. Only snow divides us. He is a young man in his mid-30s, with what I might call a Ringo Starr haircut. He has an easy smile, a gentle voice, and is unnecessarily timid about his more-than-adequate English. True, he is in a minority of German scientists who have not done a postdoctoral year in the United States. The center shifts; there was once a time when every U.S. chemist went to Germany; now they come here. Twenty-two German postdoctoral associates—or postdocs—have spent a year or more in my group.
Ansgar gives a lecture about his work. The talk is not about what he did his doctoral research on, but what he does now, crystallography. I know about crystallography by “osmosis” because I got my Ph.D. in the lab of a great crystallographer, William N. Lipscomb. I did not do crystallography; I was simply in daily contact with clever people who were learning and practicing the technique. I also knew of it out of necessity, because in my work as a theoretician explaining molecular shape, I have had to make judgments as to which seeming structural anomalies are worth pursuing, and which are to be disbelieved.
What I will say next about crystallography is not anything a crystallographer would say. When the experimental technique was difficult and a crystal structure took a year to do, there was no problem; everyone wanted to have a crystallographer friend. As crystallography became easier, an almost routine technique, the field went in search of a raison d’être. Some practitioners took on complexity—proteins, for instance. Others looked at large groups of molecules for trends and regularities (these researchers I especially value; I have written previously about them (“Crystal Cloudy, Crystal Clear,” American Scientist 86[1]:15–18, January–February 1998). Such crystallographers do just what I did in my theoretical work. Still others got the technique better and better, so that they could see not just where are the atomic nuclei and electrons near them, but also where in space reside the chemically important electrons involved in bonding and reactivity. Such a direction brings this subset of crystallographers in contact with theoreticians, who compute such things.
This is exactly the research being done both in Ansgar’s group in Berlin and in the one he is visiting in Buffalo. For my own prejudiced reasons I’m not too crazy about the work, but I won’t bore you with my prejudices. Suffice to say that when Ansgar visits Cornell, I give my guest a politely hard time during his seminar. He handles it well, as he does a pretty disconnected set of questions from the only two professors who find time to come to his talk, one of two that day, six that week. The rest of the audience is students who, as usual, sit quietly.
A Molecular Tetrahelix
At dinner that evening, over a bottle of Corbières, Ansgar tells me part of his story. He always loved chemistry. And he always read; German literature was close to him. Originally from Cologne, Ansgar did his Ph.D. at the Free University of Berlin in the group of Hans Hartl.
Barbara Aulicino
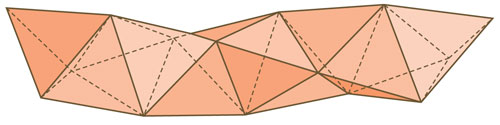
Now that was a name I knew well. Hartl and his students had made some copper compounds whose shapes had two, three or four tetrahedra that shared faces. I saw these once and thought, hey, why not an infinite chain of face-sharing tetrahedrals? David Nelson, a physicist at Harvard who once had been a student of mine, came up with the same structure in a different context, and Chong Zheng, a brilliant student fresh out of China, set to work figuring out for which elements might such a structure be stable.

Photograph courtesy of Art Tower Mito, Japan.
We thought we were original—until one of us saw a sculpture by Ted Bieler in front of the Marathon Realty Building in Toronto, with three such helices passing by each other. And then we saw Arata Isozaki’s 100-meter tower in Mito, Japan.
It looked like we weren’t that original. Actually, neither were the architects and sculptors (but they didn’t need to write footnotes, as we did), because this “tetrahelix” was the centerpiece of a chapter in Buckminster Fuller’s Synergetics!
In time, Hartl and his coworkers made the molecule, a copper iodide compound. It was as we had predicted; 12 orders of magnitude smaller than Isozaki’s tower, there it was.
Goethe and Zinc Iodide
But I have been mixing my story with Ansgar’s. On finishing his Ph.D. on zinc and cadmium halides (making them and using crystallography to determine their structures), Ansgar went to work for a couple of years in a paint and coatings company, which he didn’t like. At least the company, which will remain nameless, provided the setting for one of his detective stories. I hope there were no bodies in industrial paint mixers in real German companies.
Meanwhile, Ansgar’s literary-tour business grew into a moderately successful enterprise. For a time after I met him, he was a part-time researcher—a different path indeed in a profession addicted to a permanent search for the new, and that is a time-consuming, addictive search. To remain part-time in research Ansgar had to find a sympathetic group leader—a professor who would recognize that another obsession shared the mind of the talented young scientist and give Ansgar half of his time for his literary activities. Peter Luger in Berlin did it. However, today (in 2010) Ansgar devotes all his time to Literarisch Reisen.
Barbara Aulicino
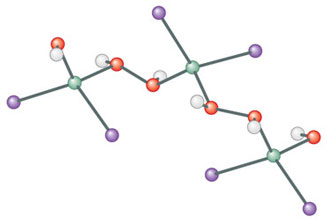
But back to our first meeting, at Cornell in 2002. At dinner, we talk of why he doesn’t “do” Goethe (as everyone else does), and we talk of Caro, Kleist, Borodin and von Arnim. In the middle of such literary talk, Ansgar says: “You know, what I’d really like to do is to go back to something strange we found for zinc iodide.” He tells me a reaction he once ran in Hartl’s laboratory, with zinc iodide (ZnI2) and water. Out of the yellow solution came one crystal, a long, colorless needle. They “stuck it in a diffractometer,” and what emerged was a structure of an inorganic polymer (right).
The ZnI2 in the structure is unexceptional; it came into the mixture as a reagent. But where did the HOOH, hydrogen peroxide, a potent bleaching agent, come from? From the water, to be sure. But what oxidizing agent, puller of electrons, could be there, to take electrons out of the OH– part of water and make the OH in HOOH? We were both chemists; the same question occurred to us, as it would have to Primo Levi, or as it will to every future chemist.
Barbara Aulicino
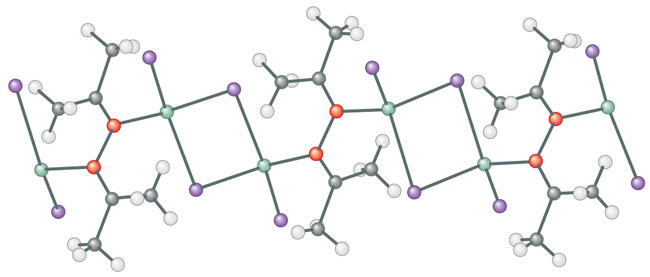
Ansgar doesn’t know. He has also run a similar reaction in acetone, the common solvent we see as nail-polish remover. Acetone is CH3COCH3. They got a linkage of the acetone units through the oxygens, and a polymeric structure through bridging with Zn2I4 (above).
This result is still more remarkable. “I’ve never seen anything like that coupling,” I say. The central coupled acetone unit, (CH3)2C–O–O–C(CH3)2, should be a very reactive species. I begin to write mechanisms and orbitals on the paper tablecloth conveniently supplied at this favorite restaurant. Chemists cover napkins with drawings of molecules; you can always tell where they’ve sat.
Now comes the tragedy. Ansgar says one student was able to repeat the synthesis. “But then it shut down,” he says plaintively. I am not an experimentalist, but I know exactly what he meant. I know the feeling in another context—the words falling in place after the seventh draft of a poem; an inkling of an orbital explanation. It’s not a gift, it’s a portal we open ourselves. It opens to the one poet in us all, as Reiner Maria Rilke wrote to Marina Tsvetaeva. Or to the one scientist who understands. And then the universe takes a jog, our attention snaps, we’re out of the flow. It shuts down.
Hans Hartl, Ansgar’s mentor, gives us more detail:
Waiting for ZnI2 that we had ordered, we used some available ZnI2 from our own chemical inventory. The compound had been bottled in a small glass flask and stored many years before. This ZnI2 sufficed only for the first experiments, which produced some crystals of the mysterious compounds. However, it was not possible to synthesize these compounds with ZnI2 bought or produced in our lab. We tried all sorts of experiments, for example storing the ZnI2 in the dark, or placing it on a window with sunshine, UV irradiation and so on. For many years we regularly repeated our attempts, but all were in vain. Thus we cannot publish these compounds.
Has Ansgar made a species that immediately went extinct? An instant fossil? Maybe. Days later I ask a talented German postdoctoral associate in my group, Beate Flemmig, to do a calculation (that’s our métier) on Ansgar’s molecule. No matter what Beate does, the strangeness of the acetone coupling does not go away. She then has the bright idea of trying, instead of a C–O–O–C linkage in the polymer, a C=N–N=C. The bonding is now normal, and the geometrical parameters fit Ansgar’s compound.
Ansgar thought that the plausible C=N–N=C linkage could come from the reaction of acetone with hydrazine (H2N–NH2). But where did the hydrazine come from? That remains a mystery. One day someone will reinvestigate the reaction, and we will learn.
Angels in Germany
We go back to my office after dinner. There is an angel to be found, a sick student to worry about, and a lecture to write.
The angel: Carl Djerassi and I have written a play, Oxygen, which had its German premiere the previous year in Würzburg. Ansgar describes his visit to the play:
I arrived there just after you left, on Tuesday. The officials told me tickets were gone—no chance to get one. So I went to the box office in the theatre and got the same answer: no chance—sold out. But I stayed there, maybe about ten minutes, and a blond-haired angel appeared. She asked, “Do you want a ticket?” I said, “Thank you, you’re an angel.” She then said with a smile that I would meet her that night—and she was a quite attractive person. At night, shortly before the performance started, I looked around in the auditorium for the angel. I saw Carl Djerassi (my first impression of him: a Hemingway of science) who was some seats behind me. The performance began and I recognized my angel: She was playing Madame Lavoisier.

Everett Collection
I must say that when I first came across the word angel in his email, I thought of another angel, one in Berlin: The angel who becomes a man in Wim Wenders’s classic dramatic film Wings of Desire, in order to experience human love and emotion.
Ansgar’s angel was female. I knew her, but I did not know her address or contact information. But I was sure that the director of the Würzburg production would be able to put the young people in touch. How could an angel not be in favor of love?
When Ansgar comes into my office before dinner, a Cornell police officer is there talking to me. A former student of mine has had psychological problems for a while. Troubled communications from him had crossed a line the day before; a threat was made. The police officer is there to talk about it. This also is a part of my life. Maybe it will find its way into Ansgar’s next Krimi.
We talk into the night, of Hans Bethe and his father-in-law P. P. Ewald, one of the great figures in crystallography. Also of Hans Hellmann, perhaps the first German theoretical chemist, who was executed in the Soviet Union in 1938 (that’s another story). And of that strange structure again.
But I have to send Ansgar home to his hotel. For the next morning, there is a class to be taught—a “World of Chemistry” general-education course, science for the citizen. The class tells young people about chemistry, its connections to culture, how chemists think. I should have told them Ansgar’s story. But I didn’t have the courage; I spoke of water instead.
©Roald Hoffman
Acknowledgement
I am grateful to Ansgar Bach for telling me his story, to him and Hans Hartl for allowing me to quote from their correspondence, and to Beate Flemmig for the calculations mentioned in the text.
Bibliography
- Literarisch Reisen. www.literarisch-reisen.de
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.