Amplifying with Acid
By Fenella Saunders
More carbon dioxide in the atmosphere means a noisier ocean
More carbon dioxide in the atmosphere means a noisier ocean

DOI: 10.1511/2010.83.121
Carbon dioxide has gained notoriety as a “greenhouse gas”; it’s one of the major waste products from human industrial activities that contribute to climate change. However, the gas that we release into the atmosphere is also absorbed into the oceans at a rate of about a million tons per hour. Seawater reacts with carbon dioxide to form carbonic acid, decreasing the pH of the oceans. This outcome has its own environmental impacts, such as damage to coral reefs and aquatic-animal respiration, but it also has a secondary consequence: It decreases the ocean’s ability to absorb low-frequency sound.
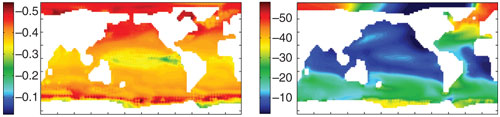
Images courtesy of Tatiana Ilyina, Richard Zeebe, Peter Brewer and Nature Geoscience.
Oceanographers Tatiana Ilyina and Richard Zeebe of the University of Hawaii, along with geochemist Peter Brewer of the Monterey Bay Aquarium Research Institute in California, report in the December 20 issue of Nature Geoscience that lowering the pH of the ocean by 0.6 units could decrease underwater low-frequency sound absorption by more than 60 percent. “Ocean acidification is not only affecting the chemistry of the ocean, but it also affects the basic physical properties,” says Ilyina.
The ocean surface’s average pH is currently estimated to be around 8.1, and to have dropped from about 8.2 since around 1800, before the industrial revolution took off, says Zeebe. A reduction of 0.1 units does not sound like much, but pH units are on a logarithmic scale, so a drop of one unit corresponds to a tenfold increase in acidity.
Using projections of fossil-fuel CO2 emissions over the next century from the Intergovernmental Panel on Climate Change (IPCC), the researchers calculated changes for seawater pH at the surface and at a depth of 1 kilometer, along with the corresponding changes in sound absorption at several frequencies below 10 kilohertz. In the IPCC’s “moderate” scenario, in which CO2 emissions remain at a constant level, pH drops by about 0.6 units at the ocean’s surface, and by about 0.2 to 0.4 units at depth.
The corresponding lowering of sound absorption depends on location and frequency. At a frequency of about 200 hertz, the drop ranges from about 10 to about 50 percent. Across all frequencies, the change is largest in the polar regions, because the colder water absorbs more CO2 and thus has a greater pH change.
Changes in pH can impact the deep ocean because at about 1 kilometer down, the properties of temperature and pressure combine to produce a “channel” of water in which sound can propagate for many thousands of kilometers. Whales and other marine life make use of this channel for long-range communication. Most human-made ocean noise forms at the surface, but it can reflect and refract down into this channel as well.
Although the vast majority of sound loss in the ocean is due to distance, reflections and turbulence, the pH- dependent component of the ocean’s sound absorbance comes from resonance reactions in natural salts, namely boric-acid compounds and magnesium sulfate. The reaction is similar for both, but it’s more straightforward in magnesium sulfate, says Brewer: “The magnesium ion and the sulfate ion are attracted to each other—in human terms it’s like they’re dating—and in their normal state they exist with a single water molecule between them, like a courting couple would have a chaperone. When a sound wave comes through, it tends to squeeze that group together and the water molecule pops out, so our attracted couple just touches, ever so briefly. When the sound wave passes by, the water molecule jumps back in and separates the pair. And the work done to do that robs the sound wave of some energy.” The problem is that as the ocean becomes more acidic, the ionized form of borate decreases, so there is less of the salt form to resonate and absorb sound.
Brewer emphasizes that this decreased-absorption effect is confined to a relatively small range of frequencies, between about 100 hertz and 10 kilohertz. He estimates that the effect will be most strongly felt around 200 to 600 hertz, over distances of roughly 100 miles. “We’re talking 40 percent of a small effect, so it isn’t a lot,” he says. “On the other hand, 40 percent is a big number in itself; if any species is sensitive in that range, they would notice the change in that scale.”
The affected range includes a large proportion of the frequencies used by marine organisms. Also, most human-generated ocean noise is in the range of 10 hertz to 1 kilohertz, and the volume is rising: The biggest component is shipping, and the number of ships worldwide has approximately doubled over the past 40 years. The researchers calculate that there could be “acoustic hotspots” that are most sensitive to changes in sound propagation, such as areas at the more extreme latitudes that also experience a lot of shipping.
“This effect has been off the radar screen, so to come along and say ‘hey, what about this’ is important,” says Brewer. “It means there are new ways of looking at the Earth, it means we are nowhere near to running out of things that are going to change. We’re fairly far down this greenhouse-gas road, and we’re nowhere near to knowing what’s going to happen to us. It’s a strange new world we’re getting into.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.