The Home of Blue Water Fish
By John Richert, Salvador Jorgensen, Abbott Peter Klimley
Rather than singly inhabiting the trackless ocean, pelagic fish species travel together in groups, which migrate between hidden, productive oases
Rather than singly inhabiting the trackless ocean, pelagic fish species travel together in groups, which migrate between hidden, productive oases

DOI: 10.1511/2005.51.42
More than two decades ago, I (Klimley) pressed my mask against my face, took a deep breath and flipped over the edge of a small Mexican fishing boat into the Gulf of California. The spectacular vision I saw that day has shaped the questions that motivate my research career in marine biology.
I was looking for hammerhead sharks over the Gorda Seamount, a shallow underwater ridge at the mouth of the gulf between the Baja Peninsula and the western coast of Mexico. Wearing a mask, snorkel and fins, a local colleague and I saw, through the dispersing bubbles from our entry, a stunning sight—we were in the middle of a swarm of fish, as if we had joined the piscine version of rush hour at a subway station. More than a hundred hammerhead sharks, some close enough to touch, passed by us as we floated above a seemingly endless, tightly packed school of flashing, silver and black skipjack tunas. Nearby, a cyclone-shaped school of gray-striped mullet snappers, each almost a meter long, swam slowly in a circle. Small green jacks and plate-shaped pompano were everywhere, darting to feed on tiny, shrimp-like krill and tail-beating larvaceans.

Amos Nachoum/Corbis
It was a wonder. But what left us dumbfounded was the sudden eruption of this multilayered community. Just one week before, we had visited the same site and seen nothing. The difference between the visits was like comparing an empty stadium to one crowded with tens of thousands of cheering fans. Had we witnessed the arrival of a massive influx of oceanic species to the Gulf of California?
Thanks to the popularity of nature shows on television, most people know that many terrestrial animals migrate from one place to another as the seasons change. For example, in Africa every year hundreds of thousands of wildebeests, gazelles and zebras leave the southern plain of the Serengeti to avoid the dry season. Without rain, the lakes evaporate and the grass dies, causing the base of the food chain to collapse and forcing large herbivores to walk hundreds of miles in search of forage. As they slowly make their long-distance trek, the herds linger at remaining water holes to sate their thirst and feed on the lush riparian foliage. These oases are terrestrial biotic "hotspots" along a migratory route with few other sources of food. When the rains return, the animals go back to their green pasture in the south.
Biologists know much less about the migration of marine species, particularly those pelagic or free-swimming fish that inhabit the blue ocean far from the coast. Animals in the pelagic realm are typically independent of the bottom and are wide-ranging. For example, fish tagged in temperate waters during the summer have been recaptured in semitropical or tropical waters during winter, and individuals tagged on one side of the ocean have later been caught on the other side. However, these data do not tell marine scientists whether the individual moved alone or as part of a school, as a single species or within an aggregation of many species. These unanswered questions are part of a general ignorance that has hindered efforts to maintain healthy populations of pelagic fishes, many of which are in a precipitous, worldwide decline because of over-harvesting. Consequently, many fisheries managers and conservationists now favor the creation of protected habitats, similar to game preserves, to ease pressure on hard-hit fish stocks. The problem with these "marine protected areas" is where to put them. What is the habitat of pelagic fishes such as tuna, dolphinfish and mackerel? Is it the broad expanse of the oceans, which cover four-fifths of the globe?
As marine ecologists, we propose that pelagic species might instead pass quickly through the vast, mostly empty ocean yet stay longer at biotic oases to feed on locally abundant prey—analogous to the way terrestrial species congregate at water holes along their migratory path. If this hypothesis is true, policy makers could focus on sheltering some of these locations, rather than the entire ocean, to improve the health of pelagic populations.
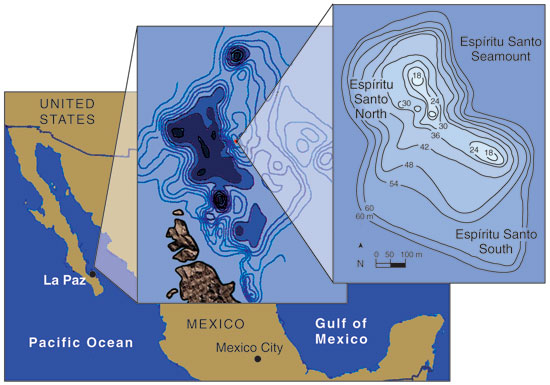
Much of our research into the ecology of pelagic fishes is based on observations in the Gulf of California, particularly at the fertile Espíritu Santo Seamount, which we have studied in concert with several Mexican colleagues. El Bajo Espíritu Santo or EBES (literally, "shoal, or bank, of the Holy Spirit" in Spanish) is a submarine ridge that, in less than 2 kilometers, rises steeply from a 1,000-meter basin to within 18 meters of the surface. We use a global positioning system to locate the area, which is completely underwater and invisible from the boat.
Tom Dunne
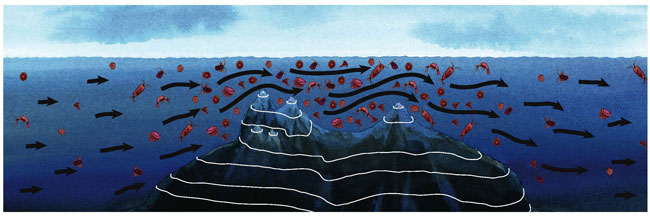
Shallow seamounts, such as Espíritu Santo and Gorda, support rich stocks of pelagic fishes because of an abundance of plankton that attracts consumers. So why is plankton (and the rest of the food web) enriched near EBES with respect to the surrounding ocean? We think that much of the answer, especially at Espíritu Santo, has to do with the so-called "Venturi effect," which describes how flow speed increases when a fluid is forced through a narrow area. This physical law also explains the high winds through mountain passes. At El Bajo Espíritu Santo, the same volume of water carrying a given number of plankters must flow through the more constricted space between the seamount and the ocean surface, providing more drifting prey over time for predator fish lurking near the peak.
Tom Dunne
Many observers have noted high biological productivity around seamounts and islands—a phenomenon that oceanographers refer to as the "island-mass effect." Part of this abundance can be explained from a purely physical perspective: Obstacles in the path of a moving fluid usually cause hydrodynamic disturbances—eddies and vortices—in the flow. Being situated in an inland sea, EBES lacks a strong unidirectional current, but it does have a daily tide that oscillates around the seamount like a cocktail swirled by a fixed stirring rod. Armando Trasviña-Castro, a physical oceanographer at the Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE) in La Paz, Mexico, estimates that tidal flows exceeding 0.5 meters per second, which can occur during spring tides, may cause twin eddies as large as 1 kilometer on the down-current side of EBES. This motion disrupts the pycnocline, the boundary between the warmer, mixed surface layer and the colder, unmixed (but often nutrient-rich) layers below. The nutrients in the upper layer enhance the growth of phytoplankton (microscopic plants), and the eddies may also trap plankton in their reversing current flows.
Rogelio Gonzalez-Armas studied plankton dynamics at EBES as a graduate student at the Centro de Investigaciones Biológicas del Noroeste (CIBNOR), also in La Paz. By towing a cylindrical net at six stations around the seamount, Gonzalez-Armas observed a two- to seven-fold increase in the concentration of copepods—minute crustaceans that feed on phytoplankton—at the ridge, compared with samples taken 10 kilometers to the north or 8 kilometers to the south. Likewise, he found three times as many chaetognaths—another type of zooplankton that eats copepods—in the water over EBES. This empirical evidence supports the conclusion that zooplankton are concentrated by the Venturi effect and retained within tidal eddies near the seamount. Together, these physical processes result in more food at the lower levels of the food chain, which in turn supports larger populations at higher trophic levels. In fact, as you approach the seamount, the texture of the ocean surface changes from perfect flatness to rippling wavelets caused by huge, diffuse schools of small fishes that dart just under the surface as they gorge on concentrated plankton. This throng of fish splits and merges abruptly as predators such as dolphinfish or wahoo chase after them.
For the past five years, two of us (Jorgensen and Richert) have studied the ecological relations among the fish assemblage at El Bajo Espíritu Santo for our Ph.D. research. Our work focuses on the movements of fishes that cause seasonal changes in the mix of species (Jorgensen) and the feeding, or trophic, relations among these fishes (Richert). We make many of our observations using SCUBA equipment, which allows us to swim a 500-meter stretch of the ridge that includes its apex. During a typical dive we see loose schools of fish dashing after plankton, which fills the water. We also see oval-shaped jacks and bullet-shaped mackerel. At the pinnacle of the ridge, only 15 meters across, female and male creolefish rush up from the bottom to release enveloping clouds of gametes. A flat plateau of sand and rock separates the peak from a second high spot to the north, a 30-meter-wide mesa made of enormous, pillow-shaped stones. A tightly packed school of large snappers and a cadre of 15 to 20 hammerhead sharks often hover nearby.

Photograph courtesy of Jessica Taylor
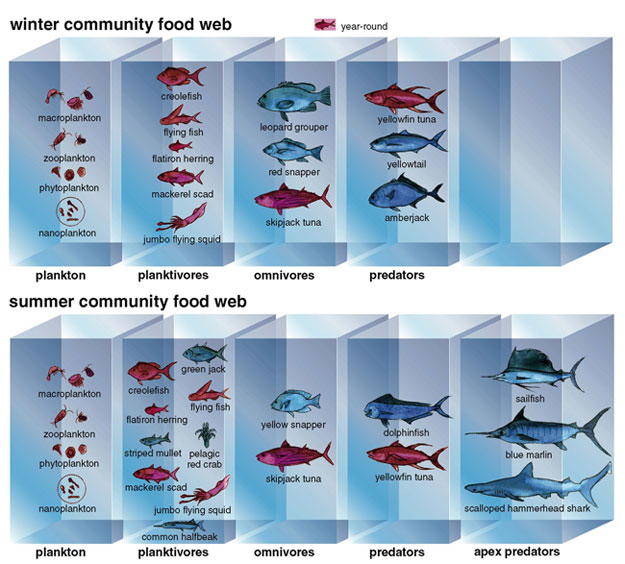
Although some species are year-round residents of EBES, others change with the seasons. We have identified at least 24 species of pelagic fishes at different times. They fall into five groups: sharks, billfish, tunas, jacks and snappers. The billfish family, Istiophoridae, is an example of the diversity at this site. Four species, three marlins and a sailfish, frequent the seamount during the day. Unlike marlins, which have relatively small dorsal fins, sailfish have a dorsal fin that stretches along two-thirds of the animal's body. Striped marlin and sailfish frequent EBES from spring through fall, but the larger, rarer blue and black marlins usually arrive in late summer. They tend to loiter close to the surface, maintaining their position by slowly sculling with their pectoral fins. They also tend to be unfriendly: On encountering a diver, a billfish will often put on an aggressive display by extending its fins to appear bigger, opening and closing its jaws and shaking its rostrum to emphasize its formidable armament. At this point it is prudent for a diver to swim away at the maximum angle of escape to avoid a charge from the fierce fish.
Tom Dunne
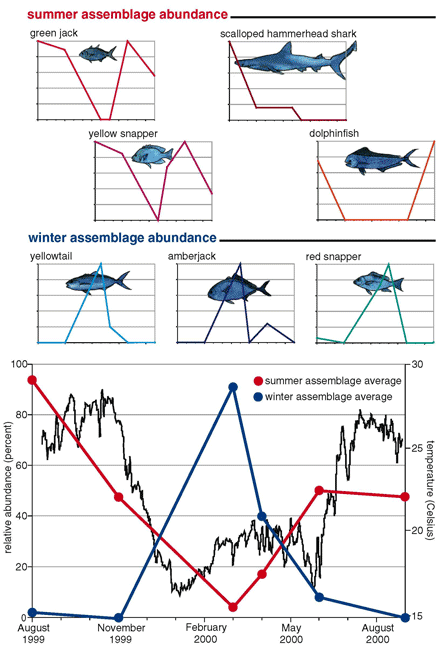
Our (primarily Jorgensen's) observations at EBES point to two different assemblages that change over the year. In the summer, when the water temperatures range from 24 to 26 degrees Celsius, a great number of fish appears, including green jacks, scalloped hammerhead sharks, dolphinfish and yellow snapper. The sailfish and marlins are also members of the summer assemblage, observations that are confirmed by catch data from the fishing community. However, none of these species is present during the winter, when the water cools to between 16 and 20 degrees. During these months, yellowtail, amberjack and red snapper colonize the seamount.
Tom Dunne
Using the identities and residence patterns of fish species as a starting point, we next sought to describe the "interactions" (a euphemism for who eats whom) among the living components of this ecosystem. Most of what scientists know about the feeding habits of deep-water fishes comes from the study of individual species that range over a wide area—an approach that overlooks the ecological connections between species. Our research (and Richert's Ph.D. thesis) has tried to fill in some of these gaps by examining trophic links between multiple pelagic predators and prey that live in close proximity within a single pelagic habitat, such as EBES. Most of the data for this project come from comparing the gut contents of fish caught at seamounts in the southern Gulf with those of fish from the open water between pinnacles. We can also assess the animal's trophic position in the food web by the ratio of nitrogen isotopes within its muscle tissue: The ratio of nitrogen-15 to the more common nitrogen-14 rises by a discrete amount with each successive link in the food chain. This method allows us to test the hypothesis that more trophic levels exist at seamounts during the summer (when more species are present) than during the winter.
Ultimately, these trophic studies will encompass not only vertebrates but also planktonic invertebrates. The traditional belief is that seamounts support longer food chains than the surrounding open water because of higher primary production (photosynthesis by phytoplankton). In the case of El Bajo Espíritu Santo, this assumption may not be true. In general, primary production is limited by the availability of nutrients, and at seamounts with a constant current, long-term eddies retain nutrients, thereby enabling phytoplankton to feed, grow and reproduce through multiple generations. However, at EBES the reversing tides make temporary eddies that cannot support multiple generations. Thus, the Venturi effect probably causes the increased zooplankton concentration that sustains the diversity and abundance of secondary consumers.
A visual census and dietary analysis are traditional ecological tools for investigating fish communities. However, given the limitations of a visual census (relatively poor visibility, mobile subjects), we also use ultrasonic telemetry. Our group, in collaboration with fisheries ecologist Arturo Muhlia-Melo from CIBNOR, keeps two electronic listening stations at the seamount to detect fish carrying beacons implanted within their bodies. Each beacon emits an ultrasonic signal with a unique code, which lets us describe the residence patterns of individual members of the assemblage.
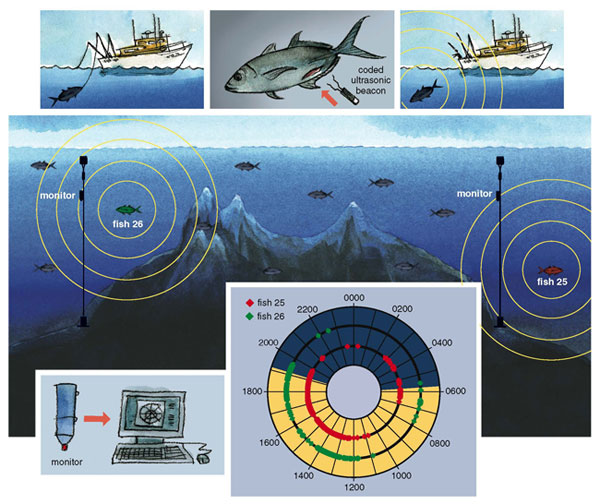
Tom Dunne
The monitors, which are moored at each end of the ridge, include a hydrophone (underwater microphone), an ultrasonic receiver and an electronic recording device within a cylinder about the size of a wine bottle. We also attach small temperature recorders to the monitors to log hourly water temperatures. (We protect these devices with the small black cases from rolls of 35-millimeter film because unique vandals in the pelagic realm—bioluminescent squid—are attracted to the intermittent flash of the light-emitting diode and will bite through the clear plastic cover.) Each outpost is weighted with an anchor (we use a heavy, metal hawser salvaged from a freighter) connected by a chain to a mid-water set of buoys and another, higher, cluster of buoys placed where they can be seen by a swimmer at the surface. The device records the identity of every beacon within 500 meters, so between the two stations we can detect any tagged individual swimming across the seamount. We (Jorgensen and Richert) periodically make a SCUBA dive to remove each monitor and temperature logger, carry them to the surface and connect them to a laptop computer to download the files of fish attendance and water temperature.
Tom Dunne
Our goal is to eventually tag fishes from all trophic levels, including consumers, predators and apex predators, in both the summer and winter assemblages. The tagging process, for some fishes at least, is fairly straightforward. For example, the green jack, a secondary consumer, and the yellowfin tuna, a predator, can be caught by hook and line. In less than two minutes, we can make an incision in a fish's ventrum (belly), insert a transmitter into its body cavity, close the opening with sutures and release the fish back into the sea.
Green jacks feed on macroplankton (such as chaetognaths), krill and larval or juvenile fishes. Although this species can be found at EBES at all times of the day or night, ultrasonic tracking data have also shown more than one individual leaving and returning to the seamount at the same time of day, indicating that they traveled together as part of an aggregation of fishes. We have also seen yellowfin tuna near the ridge at any given hour, and several tunas also moved in and out of range at the same time, showing that they were part of their own school. The period during which the greatest number of tunas were present (roughly 7 a.m. to 7 p.m.), overlaps with our preliminary data on the peak residence times of green jack fishes (about 10 a.m. to 8 p.m.). Are the tuna preying upon the jacks during these overlapping periods? Or do the species share some migratory association? By integrating our analyses of feeding habits and residence patterns for multiple species, we hope to answer these important questions.
Compared with the relative ease—even leisure—of reeling in jacks and tunas to tag, placing beacons on hammerhead sharks is a daunting challenge. Unlike the other two species, scalloped hammerheads do not feed at the seamount, preferring to bide their time in small schools at greater depths. For that reason, we have to swim down to the sharks and use a pole spear—a long, flexible rod powered by a stretchy loop of rubber tubing—to attach the tags. If the sharks are in less than 25 to 35 meters of water, one of us (Klimley) can take a deep breath at the surface, free-dive to the school, pick out a large individual and release the spear so that a small, barbed dart on the end penetrates the tooth-like skin of the shark's back. The dart is connected by a short tether to the ultrasonic beacon, which floats unobtrusively just above the back. When the sharks are in deeper water or smaller schools, we use a type of SCUBA called a closed-circuit rebreather, which doesn't emit bubbles to spook the fish, and wait silently for the sharks to approach. Once tagged, the shark usually accelerates away for a moment, but quickly returns to the group, indicating that the stress from the procedure is minimal.

Photograph courtesy of the authors
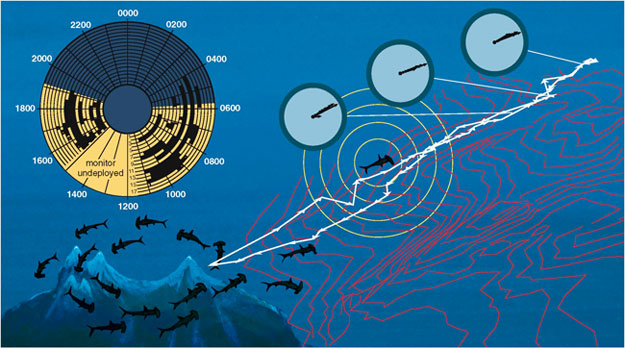
As is true for the other tagged species, some individual sharks appear to move independently and others swim together as a school. However, the similarities end there. On the days that we tracked their movements, all of the hammerheads at El Bajo Espíritu Santo left the ridge between 6:00 and 8:00 p.m., and they all returned between 4:00 and 7:00 a.m. the next day. The uniformity of this behavior led us to wonder where these predators were going. To answer that question, we used an ultrasonic monitor on the boat to follow behind some of the tagged hammerhead sharks at night. It turned out they often made extensive trips to other nearby areas, probably to eat the squid found in deeper water. We based this inference on a pattern of slow swimming at great depths when farthest from the seamount, plus the fact that the squid in their stomachs often possessed light-emitting photophores, which are found in benthic species.
During these excursions, the hammerheads showed an extraordinary sense of direction. One shark swam for 20 kilometers in a direct line away from the seamount, abruptly turned 180 degrees and came back to EBES along the same path. This feat was especially remarkable considering the animal remained 200 meters below the surface (eliminating celestial cues) and 800 meters above the bottom (obscuring topographical landmarks). Indeed, when that shark was at its greatest distance from the seamount, the consistency of its navigational headings (indexed by a so-called "coefficient of concentration") measured 0.996—a deviation of only 0.4 percent from a perfectly straight course. In short, this hammerhead was swimming with the precision of an automobile driving down a highway—without being able to see the striped lines or even the road itself.
Knowing the unique sensory capabilities of the hammerhead (it detects faint bioelectric fields, which help it find hidden prey), we later surveyed the geomagnetic field around the seamount and discovered that the paths of the sharks coincided with magnetic valleys (intensity minimums) and ridges (intensity maximums) leading away from El Bajo Espíritu Santo like spokes from a hub. These magnetic traces were likely produced by the flow of magnetite-impregnated basalts from the seamount during volcanic eruptions. Taken together, the evidence suggests that this predator, unlike some others, does not congregate at the seamount because it is a biotic hotspot, but because the area provides a network of "paths" that enables it to find a constellation of productive feeding grounds in the surrounding waters.
The astounding biodiversity at Gorda Seamount that initially motivated our research is rare today. Although the Gulf remains fertile, destructive fishing practices and poor fisheries management have jeopardized many fish populations. A school of hammerheads, for example, often included hundreds of individuals in the 1980s. But after intensive gillnetting at shark nursery areas over the past two decades, it is now unusual to see schools of even 20 sharks. We often visit traditional habitats and do not see a single one.
It is easy, but incorrect, to blame only the fishers for declining fish populations. We realize that fish stocks are an important resource for coastal communities and that fisheries management must also reflect social and economic needs. Our Pelagic Fish Research Group, which includes scientists from the U.S. and Mexico, is striving to understand the ecology of these species partly so that management efforts can be guided by a more comprehensive vision of pelagic fish assemblages. If policy makers know how trophic webs and migration patterns operate at and between seamount hotspots, they will be better equipped to design marine protected areas that are more likely to sustain the size and diversity of pelagic populations, thereby benefiting the fish, and the fishers, that depend on them.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.