Now In Color
By Robert Frederick
Even though they are far smaller than the shortest wavelength of visible light, tiny biological objects can finally be imaged in multiple hues.
Even though they are far smaller than the shortest wavelength of visible light, tiny biological objects can finally be imaged in multiple hues.

DOI: 10.1511/2017.124.12
Recalling the moment he and his colleagues composed their first two-color image of the structures inside an endosome (a compartment attached to the cell membrane that helps sort incoming substances), Stephen Adams says his initial response was, “Wow, this is a really pretty picture. I wonder what it means?”
Adams, a biochemist at the University of California, San Diego, had been working on the project for 13 years. “Thirteen sounds unlucky, so I like to round up,” Adams says. It started just after a Christmas holiday during which his longtime colleague Roger Tsien had spent some quiet time thinking about how an electron microscope could image biological samples in color. “He would just pick up concepts from completely different fields,” Adams says, “and then he’d think ‘Well, what chemistry do we need to achieve this?’”
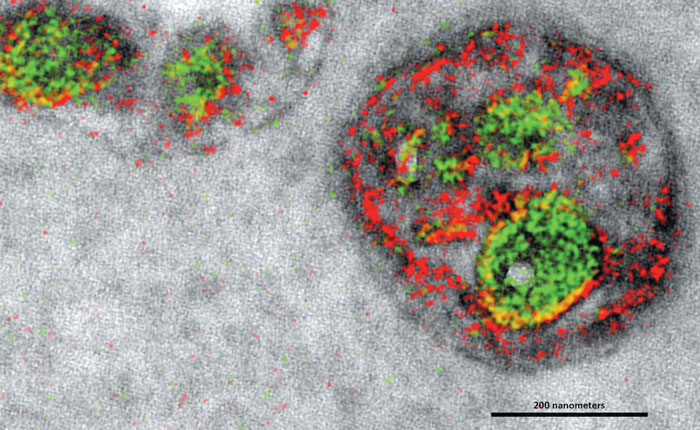
Stephen R. Adams et al.
Individually coloring an object’s parts makes it far easier for us to understand how the whole object functions. But even though electron microscopes allow us to see at resolutions millions of times better than that of our eyes, they can’t distinguish where different proteins are located in a cell, which is necessary to correctly colorize them. So Tsien’s idea was to chemically tag specific proteins with metals, providing the electron microscope with a distinctive signal. Tsien, who died in August 2016 at age 64, won the Nobel Prize in Chemistry in 2008 for his developments with green fluorescent protein, which is used to tag different proteins with multiple colors in living creatures. This work to colorize electron microscope images built on that knowledge. But the team had to work out a complicated process to tag multiple proteins in biological samples that were headed to the vacuum environment of electron microscopy. As Adams explains, “That is what took us so long.” The team reported their work in the November 17, 2016, issue of Cell Chemical Biology.
“The trick is that we generate a polymer at the site of each protein,” Adams says. Then the biological sample is placed in a solution, and, one by one, different metals are washed over the sample and precipitate out when they attach to a polymer at the site of the specific protein they are meant to tag. Afterward, under the electron microscope, the deposited metals cause distinctive spectra, which are used to identify their locations: Peptides taken up by the endosome were labeled with the metal praseodymium (falsely colored red, above). Cerium-labeled proteins (green) show the location of proteins that start on the outside of an endosome and then get internalized as “it does this unusual, inward budding of the membrane,” Adams says.
“Of course, people haven’t been able to look at [the internal structure of the endosome] in such high resolution before,” Adams says, “so it’s not like I could just say ‘Ah yes, it’s exactly as we expect.’” So like with any proof-of-concept technique, researchers will repeat their work, refine their processes, improve multicolor electron microscopy, and look for still other ways to verify what we can now see.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.