Deep-Ocean Life Where Oxygen Is Scarce
By Lisa Levin
Oxygen-deprived zones are common and might become more so with climate change. Here life hangs on, with some unusual adaptations
Oxygen-deprived zones are common and might become more so with climate change. Here life hangs on, with some unusual adaptations

DOI: 10.1511/2002.33.436
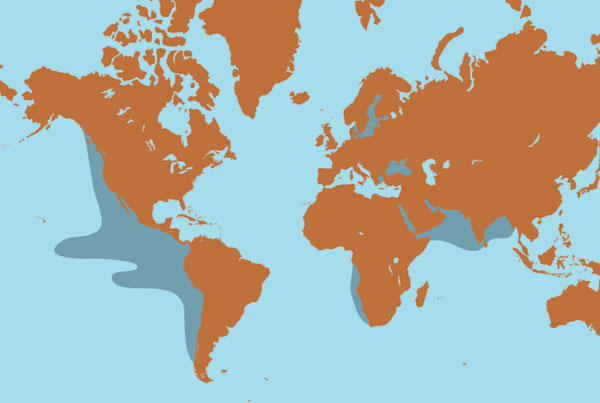
In 1925, the Meteor expedition set out from Germany to measure the ocean's depth, study marine chemistry and, on the side, extract gold from seawater. The gold project didn't pan out, but expedition data revealed a curious feature in the Atlantic, off southern Africa: a giant wedge of oxygen-deficient water 300 to 400 meters down.

D. W. Miller
The surface layers of the ocean generally obtain oxygen from diffusion and brisk circulation. This water sinks to the seafloor, supplying oxygen to deep-sea life. But sluggish circulation and oxygen-poor source waters can reduce oxygen concentrations at intermediate depths. In several oceans, this effect is unusually pronounced. Where primary production by photosynthetic life is very high, the decay of sinking organic matter consumes so much oxygen that the middle depths contain dramatically less of the gas than water above or below. Such oxygen-minimum zones, or OMZs, turn up in productive open oceans and on continental margins. They can stretch for thousands of miles and persist for thousands of years.
Barbara Aulicino
I first encountered an OMZ while studying animal communities on Volcano 7, a submerged mountain about 200 miles west of Acapulco, Mexico. The seamount has its summit 730 meters below the surface and its base 3,400 meters deep. With a group of geologists, I first visited Volcano 7 in 1984 to photograph and study the mountain's slopes using Alvin, a submersible vehicle based at Woods Hole Oceanographic Institution (WHOI), and ANGUS, a camera mounted on a sled. I noticed the fascinating sea life, but we did not measure oxygen on that trip. Four years later, I returned with a group of women who were studying plankton and life on the ocean floor. Again, we employed Alvin to explore the volcano's surface.
Barbara Aulicino
Because Alvin had space for only a pilot, two scientists and our gear, we had to squeeze down on our knees and peer sideways to look through a porthole. The vehicle's exterior lights, though powerful, illuminated only six to eight feet of water, focusing our attention on the fauna at hand rather than the larger view. At about 800 meters below the surface, dense groupings of crabs, shrimp, brittle stars and sponges were zoned like invertebrates on a rocky shoreline. But as we moved up the mountain's slope, the rocks suddenly looked barren, covered with the merest fuzz of life.
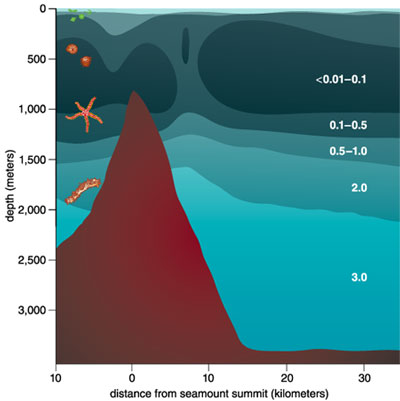
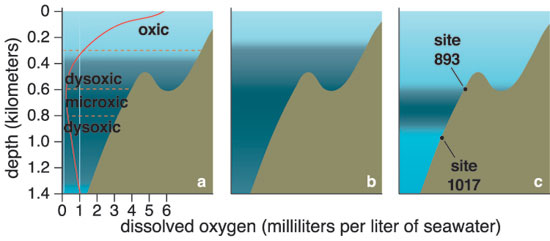
Wondering what might account for the dramatic change, we looked at the oxygen data generated by a sensor mounted on the submersible's shell. A liter of seawater can hold 7 or more milliliters of oxygen. (For comparison, a liter of air can hold 210 milliliters of oxygen.) But the water that swathes the peak of Volcano 7 contains less than one-seventieth of that amount—0.1 milliliter per liter. The seamount summit protrudes into a vast OMZ—a region of the ocean containing less than 0.5 milliliter of oxygen per liter of water. It is surprising that any life exists where the oxygen supply is so sparse.
In addition to the OMZs that lie off the western coasts of the Americas, well-established OMZs have also been discovered in the Arabian Sea, the Bay of Bengal and off the western coast of Africa. In the United States, the zones lie off California and Oregon. California's Santa Barbara Basin, with its bottom at 585 meters, is right within the California OMZ and has very little oxygen. My group at North Carolina State University and then at Scripps Institution of Oceanography, in collaboration with U.S. and international investigators, has studied OMZs off California, Mexico, Peru, Chile and Oman. Using data on seafloor topography and oxygen measurements from the National Oceanographic Data Center, we recently teamed up with John Helly at the San Diego Supercomputer Center to estimate how much of the continental margin seafloor is intercepted by OMZs. Our global estimate was about 2 percent.
Seasonal oxygen-depleted zones are also known. Water over the shallow shelf off Chile becomes depleted during the spring and summer, when it is fed by the poorly oxygenated Peru-Chile Undercurrent. In fall and winter, it becomes oxygenated again as cold, sub-Antarctic currents prevail. Human activities deplete other bodies of water. In some estuaries and coastal regions, fertilizer runoff or sewage outfall promotes periodic phytoplankton blooms, which rob the water of oxygen as they decay. Such human-inspired hypoxic events have taken place in many parts of the world, including Chesapeake Bay, the New York Bight and the Gulf of Mexico. A severe hypoxic event in the northern Adriatic in 1977 slaughtered masses of marine organisms.
Understanding the formation and ecological impact of oxygen-depleted zones is critical because their number and intensity are likely to increase as global warming and nutrient enrichment rob the ocean of oxygen. Because such zones are inhospitable to animals we consider edible, their expansion could have a devastating effect on commercial fishing.
OMZs generally form where winds create currents that move water along a continent's western shoreline. Aided by the Earth's rotation, such currents sweep water offshore. Replacing the lost mass, cold water rises up from the ocean's depths. It brings nutrients, especially nitrogen, to the sunlit surface, where a lack of nutrients would otherwise limit plankton and nekton growth. When the resulting glut of surface-dwelling organisms dies, the bacterial garbage recyclers of the ocean deplete the water of oxygen as they degrade the detritus. If such conditions persist for long periods, middle depths can become permanently short of oxygen, and an OMZ can appear.

Photograph courtesy of Peter Lamont, Scottish Association for Marine Science.
Areas that lack a constant supply of oxygenated water are good candidates for OMZ formation. For example, the eastern Pacific and Indian oceans receive water from the North Atlantic, where most new deep water forms. As this water travels south for hundreds of years, it loses oxygen. The Arabian Sea, which contains a vast OMZ, also receives poorly oxygenated waters, which come from the Red Sea, Persian Gulf and Banda Sea.
Interested in the adaptations of seafloor communities to extreme environments, I began to study OMZs in 1988, when groups led by Karen Wishner (University of Rhode Island), Marcia Gowing (University of California, Santa Cruz), Lauren Mullineaux (WHOI) and myself visited the slopes of Volcano 7 aboard WHOI's research vessel Atlantis II. Because OMZs usually lie between 200 and 1,300 meters, they can intercept the seafloor on seamounts and edges of continents.
Descending through an ocean containing an OMZ, you encounter a wide range of oxygen concentrations. The first part of your journey takes you down through well-oxygenated water, which persists for 500 to 1,000 meters off the coast of Central America or Peru and for up to 400 meters off the coast of Oregon or California. In waters off Mexico, the first 100 meters is sunlit, so you see small planktonic organisms. Once you enter the OMZ, the oxygen content drops precipitously to below 1 milliliter of oxygen per liter of water, placing you in a low-oxygen—dysoxic—zone. At 300 to 700 meters, the water's oxygen content stabilizes at its lowest level, forming a microxic (less than 0.1 milliliter per liter) zone. Oxygen gradually becomes more plentiful again as you continue to descend. But if you reach the seafloor while still within the zone, you find soupy mud off Oman, sand made of shells on the slopes of Volcano 7, and fine sediment with phosphorite rocks off Peru. These sediments are rich in nutritious debris. Thioploca was first found by Chilean scientist Victor Gallardo in the OMZ under the Peru-Chile Subsurface Countercurrent system, where it colonizes more than 10,000 square kilometers of continental shelf. Like Beggiatoa, its cells form filaments. But dozens of filaments—and even filaments of different Thioploca species—share a sheath. Off the Chilean coast, Thioploca constructs thick, gelatinous mats on the seafloor that can weigh up to 120 grams—about a quarter of a pound—per square meter. Vertical mucous tunnels under the mats allow Thioploca filaments to slide down into the sediment and find hydrogen sulfide. Because zones of sulfide and nitrate do not overlap, the bacteria commute up into seawater to obtain nitrate. Swaying back and forth and emerging from their sheaths, they form a quivering, white lawn.
Heide Schulz, with an international team of scientists aboard the Russian research vessel Petr Kottsov, recently discovered an even larger sulfur-oxidizing bacterium in sediments off Namibia. Thiomargarita namibiensis has pearly spheres for cells that can reach three-fourths of a millimeter—almost twice the size of the period at the end of this sentence. These cells also grow in chains, but they are not motile. T. namibiensis holds the world record for bacterial size, being about 3 million times larger than most bacteria.
Rather than circumventing the need for oxygen, other organisms have adapted to OMZs by developing highly efficient ways to extract oxygen from depleted water. Bottom-crawling crustaceans in the genus Ampelisca have gills with a large surface area, as do OMZ-dwelling mysids (small shrimp-like animals), fishes and cephalopods. Polychaete worms have extensive long tentacle-like branchiae that presumably facilitate oxygen uptake. Invertebrates in OMZs often stabilize sloppy mud and facilitate water movement by constructing nests, mudballs or tubes.
Nancy K. Sanders and James Childress of the University of California, Santa Barbara, studied the adaptive features of Gnathophausia ingens, a large red mysid that squirts luminescent material when disturbed. This shrimp is common in OMZs. Important features include excellent ventilation, a large surface area on the gills, minimal separation between seawater and the blood flowing through the gills, the ability to remove up to 90 percent of oxygen from inhaled water, brisk interior circulation and an efficient respiratory pigment.
Unusually adept respiratory pigments appear to be an adaptation to OMZs. The blue pigment hemocyanin has more extreme properties in G. ingens from the OMZ off California than in G. ingens from Hawaii, where oxygen concentrations are higher. Its very high affinity for oxygen enables it to snatch the gas from oxygen-poor water, but other properties allow it to release oxygen readily to tissues. An unusual insensitivity to changing temperature keeps the pigment functional at different depths. The respiratory pigment hemoglobin, which colors vertebrates' red blood cells, is found not only in fishes but also in certain OMZ-dwelling bivalves, including mussels we saw on the margin off Oman.
Copepods (tiny crustaceans that function like insects of the sea) in the genus Lucicutia have fine-tuned their life cycles to different oxygen levels. L. grandis, a large red-orange species, is common in the most hostile regions of the Arabian Sea. Adults live at the deepest level of the OMZ, where the oxygen concentration of about 0.14 milliliter per liter is adequate for reproduction. The youngest offspring live farther up the water column, and the next life stage lives in the shallowest part of the OMZ, where extraordinarily low oxygen concentrations (0.069 milliliter per liter) protect them from predators. The crustacean Orchomene obtusis, which scavenges in the bottom waters of Saanich Inlet, British Columbia, moves in and out of OMZs. It takes advantage of the plentiful food and lack of predators in hypoxic waters and swims out to obtain more oxygen—a strategy dubbed "eat and run."
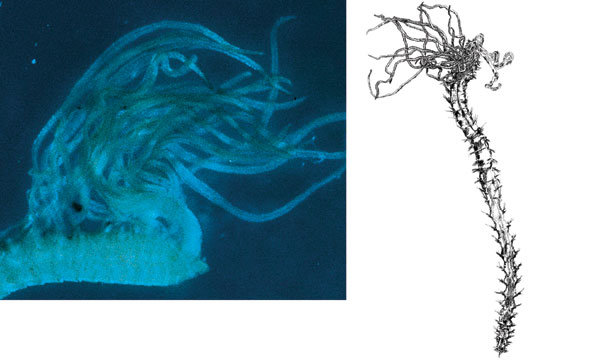
The abundance of nutrients, combined with diminished competition for food and fewer predators, offsets some of the disadvantages of OMZs for organisms that can adapt to this unusual milieu. As might be expected, these adaptations can also be highly unusual. For example, giant bacteria that use nitrate to oxidize hydrogen sulfide are conspicuous in OMZs. Their chemical expertise enables them to obtain energy without the need for oxygen. The resulting crystals of elemental sulfur accumulate, making the cells look white. Two sulfur-oxidizing bacteria, Thioploca and Beggiatoa, are common in sediments under OMZs off northern Chile, Peru, Oman, Namibia and Pakistan. Thioploca cells align in long filaments that look like angel-hair pasta. The filaments glide through the water in slimy sheaths and, in some locations, form dense, yellow mats.
Like communities of animals that occupy different habitats on dry land, communities in undersea habitats also differ in size and composition. The first scientist to study the unique features of OMZ communities in detail was Howard Sanders of WHOI. Comparing fauna dredged from marine and estuarine environments off the coasts of India, Madagascar, New England and South America, he concluded in 1968 that two Arabian Sea samples contained the fewest species. "The probable cause for these modest diversity values is the low-oxygen minimum layer found throughout the northern Arabian Sea at the 100 to 200 meter depth," he wrote. Sampling across the continental margin off Walvis Bay on the Atlantic coast of the southern African country now called Namibia, Sanders noted even less diversity at 100 meters, where the oxygen saturation was below 2 percent. Around Walvis Bay, the existence of an OMZ is betrayed by the occasional smell of rotten eggs; the smell comes from sediments permeated with hydrogen sulfide.
Now that many OMZ communities have been studied, we can point to some common features. OMZs intersected by the seafloor tend to have populations of large, filamentous sulfur bacteria, such as the species described above. Meiofauna (microscopic animals) are also abundant in OMZs, but there are limited numbers of macrofauna (animals larger than half a millimeter) and megafauna (bigger animals, such as crabs, anemones and starfish). Biologists separate these groups by shaking their samples through progressively smaller sieves. Megafauna can be recognized with the naked eye.
Small body size is one of the most obvious features of animals in OMZs. The barren appearance of Volcano 7's summit results not from the absence of life but from the lack of animals larger than a centimeter. There appears to be no simple relation between oxygen content and body size within any group of small animals, however.
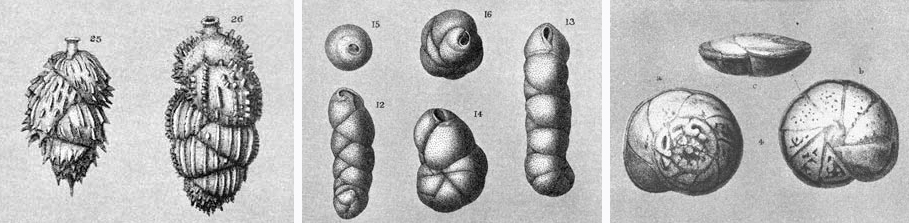
The single-celled organisms of the order Foraminifera are important members of OMZ communities. Foraminifera tend to be more abundant than multicellular animals of similar size, nematodes excepted. In contrast with metazoans that have adapted to OMZs, foraminifera have calcareous or agglutinated shells, called tests, which range in size from less than 0.1 to several centimeters. During a 1994 expedition, Andrew Gooday of the Southampton Oceanography Centre collected foraminifera from soupy mud under the OMZ off Oman. Lowering corers over the side of a British vessel, the Royal Research Ship Discovery, he sampled both the center of the OMZ, at 412 meters, where the oxygen concentration was 0.13 milliliter per liter, and a site at 3,350 meters, where it was about 3 milliliters per liter. In the top centimeter of the cores, Gooday found 25 times as many foraminifera in the OMZ samples as in the deeper samples. Ninety-three percent of the particles were smaller than 500 micrometers, compared with 66 percent of those from the 3,350-meter sample. However, the OMZ samples yielded the foraminifera with the largest dimensions. In total, Gooday counted only 70 different species of foraminifera from the OMZ compared with 366 species from the deeper site. Structurally complex, thin-shelled foraminifera called rotaliids, which are very tolerant of low oxygen, dominated the OMZ samples but were uncommon in the deep water.
Nematode worms dominate the meiofauna in OMZs, accounting for more than 95 percent of the organisms by number and by weight. These minute, simple animals inhabit dry land and fresh water as well as being the predominant multicellular animals in marine sediments. The large surface-to-volume ratio of their narrow bodies is eminently suited to efficient oxygen uptake. Thus, the density of nematodes can be several times greater at the core of an OMZ than in deeper, well-oxygenated waters. Many other meiofaunal groups—copepods, kinorhynchs, tardigrades and rotifers—are poorly represented in OMZ communities. Even so, meiofaunal density in OMZs often matches that in densely populated oxygenated regions, correlating positively with food availability or quality.
Drawing and photograph courtesy of Peter Lamont.
The density of macrofauna can be 30 percent to 99 percent lower in the core of an OMZ than in well-oxygenated regions of the ocean. The most abundant members are annelids, which tolerate oxygen depletion well, perhaps because their thin, bare bodies facilitate oxygen diffusion. These segmented worms, which tend to be surface feeders, are usually polychaetes, but sometimes oligochaetes (which have fewer appendages) can be dominant. Analyzing data from the eastern Pacific, northeastern Atlantic and northwestern Arabian Sea, John Gage of the Scottish Association of Marine Sciences and I concluded that the availability of both oxygen and organic carbon determines the richness of macrofaunal species in OMZs until the oxygen content rises to about 0.45 milliliter per liter. Above that level, oxygen is much less important.
Among the macrofauna, crustaceans and molluscs are rare where the oxygen concentration is below 0.15 milliliter per liter. Some exceptions are Ampelisca amphipods, which build parchment-like tubes in soft sediments, the gastropod Astyris permodesta and a thin-shelled mussel, Amygdalum anoxicolum, which builds a cocoon from mud. G. ingens can live at oxygen concentrations as low as 0.2 milliliter per liter. Megafauna—such as crabs and shrimp—are also rare in the heart of an OMZ, where oxygen drops below 0.1 milliliter per liter. Below the core, where oxygen concentrations are somewhat higher and nutrients still plentiful, larger animals become more common. Shrimp, crabs, sponges and brittle stars often congregate at such edges. The lower boundary of an OMZ is the region richest in megafauna; it can also be one of the richest habitats in the ocean.

Photographs courtesy of Lisa Levin.
A photographic transect down Volcano 7, which lies within an OMZ from its summit to a depth of 1,300 meters, showed only bony fish called rattails, sponges and seapens at 740 meters, where the water contained less than 0.1 milliliter of oxygen per liter. Between 750 and 770 meters, there were successive zones of crabs, shrimp, brittle stars and anemones, with very dense patches of megafauna—as many as 19 animals per square meter—at 800 to 810 meters. Below 1,000 meters, where the oxygen concentration was higher, the density fell to 0.5 animals per square meter.
Such surveys illustrate another striking feature of OMZ communities: reduced diversity. On the summit of Volcano 7, more than half the rotaliid foraminifera collected belonged to a single species. The foraminiferan Epistominella bradyana is dominant in Mexico's Gulf of Tehuantepec, Nonionella stella in the Santa Barbara Basin and Bolivina seminuda on the Oman margin. Likewise, the most abundant macrofaunal species can account for up to 83 percent of the total macrofauna in an OMZ. Although oxygen concentration appears to reduce diversity when it falls below 0.4 to 0.3 milliliter per liter, it is not the sole factor. Toxic sulfides or competitive interactions might also contribute to low diversity in OMZs.
Animals in OMZs obtain energy and carbon for growth by breaking down detritus. Surface waters are filled with phytoplanktonic organisms that obtain their energy from the sun and with animals that eat phytoplankton; when these organisms die they sink as detritus. Because sinking detritus is so abundant in oxygen-depleted waters, scientists used to assume that photosynthetic organisms stood alone as the energy source forming the base of the food chain in OMZs. Prompted by Victor Gallardo, we now wonder whether chemosynthesis, practiced by sulfur bacteria, could also play a role.
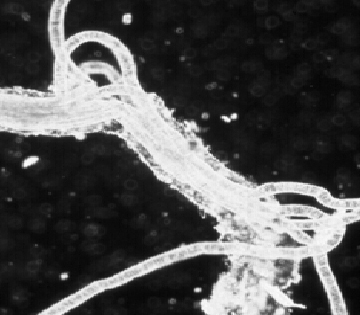
Photomicrograph courtesy of Lisa A. Levin.
In 1994, Henrik Fossing of the Max Planck Institute of Marine Microbiology in Bremen, Victor Gallardo and investigators from Denmark sampled sediment from the OMZ off Chile. After squeezing pieces of Thioploca mat, they measured nitrate concentrations. The first liquid to emerge had a nitrate content similar to that of seawater. But greater pressure released liquid with 100 times as much nitrate. Subsequent studies showed that this liquid came from the membrane-bound vacuole that occupies most of the bacterium. Astonishingly, the nitrate concentration in such vacuoles was 500 millimolar—up to 20,000 times the concentration in seawater. Thus, Thioploca stores the nitrate it needs for oxidizing hydrogen sulfide. The nitrate takes the place of the oxygen used by most organisms, and the vacuole serves as a lung. Presumably, the ability to store nitrate gives Thioploca a distinct advantage over bacteria that must have simultaneous access to an oxidant and a reduced compound.
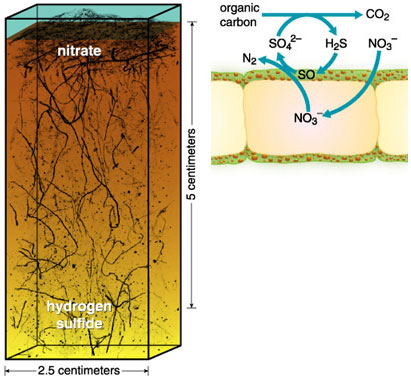
Reconstruction courtesy of Heide Schulz, University of California, Davis; drawing adapted from Fossing et al. 1995.
Hydrogen sulfide, the reduced compound used by Thioploca, is produced by sedimentary bacteria that reduce sulfate. In some locations, filaments of the sulfate-reducing Desulfonema ornament the sheath of Thioploca, creating an efficient recycling system. A more intimate arrangement has evolved in the gutless oligochaete Olavius algarvensis, which shelters two bacterial species in its cells. One bacterium produces sulfide, and the other oxidizes this product. Even in sediments completely devoid of oxygen, hydrogen sulfide is consumed rapidly. Thus, bacteria in anoxic sediments reduce sulfate to hydrogen sulfide. Whereas some sulfur-oxidizing bacteria require oxygen to oxidize hydrogen sulfide, others use nitrate, releasing energy that can be used for growth. These bacteria can be free-living, as is Thioploca, or they may live as symbionts, as do Olavius and bivalves of the family Lucinidae. When seafloor bacteria are digested by other organisms, the energy and carbon stored in their cells becomes available once more. Therefore, chemosynthesis as well as photosynthesis might initiate food chains that support OMZ animals. One of our goals for the next few years is to assess the extent to which chemosynthesis fuels oxygen-minimum ecosystems.
One of the advantages of studying OMZs is their stability, which permits observations over extended periods. But although OMZs persist for thousands of years, they do expand and contract as the global climate changes. Detecting and understanding prior alterations might help us predict—or even delay—future changes.
Climatic changes likely to have affected the oceans' oxygen inventory include the ice ages. These periods have not been unremittingly cold: Ice cores from Greenland reveal that at least 24 periods of sudden warming occurred between 115,000 and 14,000 years ago. These interstadials—warm periods between icy cold periods—took no more than a few decades to develop, and they persisted from decades to hundreds of years.
Given the impossibility of measuring the oxygen content of past waters, scientists have had to devise proxy methods. One method records the number and species of fossilized foraminifera in samples of ocean sediment. Identifying the foraminifera that currently dominate waters with known oxygen concentrations allows us to infer the concentrations that existed when the fossilized foraminifera were alive. Using this approach, Kevin Cannariato and James Kennett at the University of California, Santa Barbara, recently made detailed studies of the Santa Lucia Slope, about 60 kilometers west of Point Conception, California. After seeing how assemblages of fossilized foraminifera changed over the past 60,000 years, they determined whether the major changes correlated with alterations in global climate.
Adapted from Cannariato and Kennett 1999.
The foraminifera Bolivina argentea, Buliminella tenuata and Chilostomella ovoidea currently dominate the Santa Lucia slope where oxygen concentrations are between 0.1 and 0.3 milliliter per liter. Different foraminifera are dominant where oxygen concentrations are higher. The fossil study showed that past assemblages changed rapidly. Foraminifera typical of low oxygen concentrations were dominant during warm periods such as the interstadials and the last glacial maximum, whereas foraminifera typical of higher oxygen concentrations were dominant during the cold periods. The California OMZ presumably expanded into shallower and deeper waters during certain warm periods and contracted during cold periods. Such fluctuations over time appear to have been common along the northeastern Pacific margin and northern Arabian Sea.
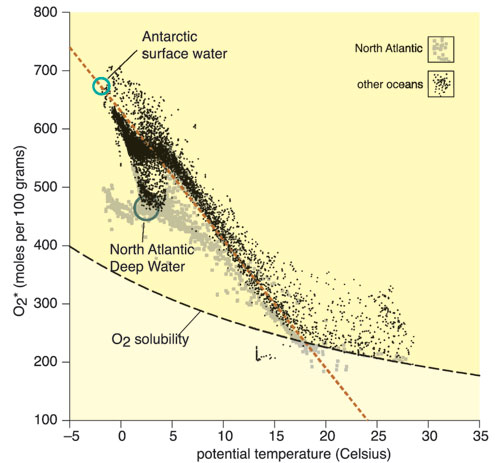
Because history tends to repeat itself, the current trend toward global warming has the potential to expand OMZs, depriving large regions of the ocean of commercially useful fauna. Computer modeling completed this spring by Ralph Keeling and Hernán Garcia, two colleagues at Scripps, suggests that the oceanic inventory of oxygen is already falling. The scientists plotted a measure of the amount of oxygen that various oceans would lose to the atmosphere as water temperature rose. The resulting slope was several times steeper than a plot of oxygen solubility against temperature. Thus, the influx of detritus into the ocean's interior, not oxygen solubility, must be the major influence on oxygen exchange. The relation between oxygen exchange and heating allowed Keeling and Garcia to estimate oxygen loss from the world's oceans for the years 1990 to 2000. The figure they derived was 2.9 trillion moles of oxygen per year. To obtain this amount, you would have to remove all the oxygen from 92 trillion kiloliters of surface seawater at 5 degrees Celsius (about one ten-thousandth of the oxygen in all the world's oceans). The calculations suggested that 18 percent of the lost oxygen came from the North Atlantic, 20 percent from the deep Southern Ocean and 61 percent from the rest of the upper ocean. The global estimate is not dissimilar to that derived using ocean general circulation models, and oxygen loss is likely to increase as human activities exacerbate global warming.
Tom Dunne
To test these models and predict the impact of global warming on marine fauna and our seafood supply, it will be necessary to directly monitor the changing oxygen content of the world's oceans. To learn more about OMZs, we will need to identify factors that control animal abundance and boost density at OMZ boundaries. Molecular, enzymatic, physiologic and reproductive studies together will allow us to solve the puzzle of how animals survive at such low oxygen concentrations. The role of chemosynthesis in oxygen-depleted zones and the contributions of OMZs to the generation of new species are among the most exciting evolutionary questions. By integrating biogeochemical, molecular, microbiological and ecological research, scientists hope to fill in some of the gaps. Given the unique features of OMZs, their findings could be surprising.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.