Ecology of Transgenic Crops
By Michelle Ann Marvier
Genetically engineered plants might generate weed problems and affect nontarget organisms, but measuring the risk is difficult
Genetically engineered plants might generate weed problems and affect nontarget organisms, but measuring the risk is difficult

DOI: 10.1511/2001.18.160
On May 20, 1999, a short article in Nature called attention to a potential ecological problem with a genetically engineered, or transgenic, crop. John Losey and his colleagues at Cornell University reported that a variety of transgenic corn could kill the larvae of monarch butterflies.
Opponents of transgenic crops held up the report as evidence of the potentially devastating environmental impact of this new technology. Proponents, on the other hand, largely dismissed this laboratory-based research as unrepresentative of conditions on real farms. Yet despite the disagreements, this study draws attention to the difficulty of determining the safety or danger of this new generation of crops.
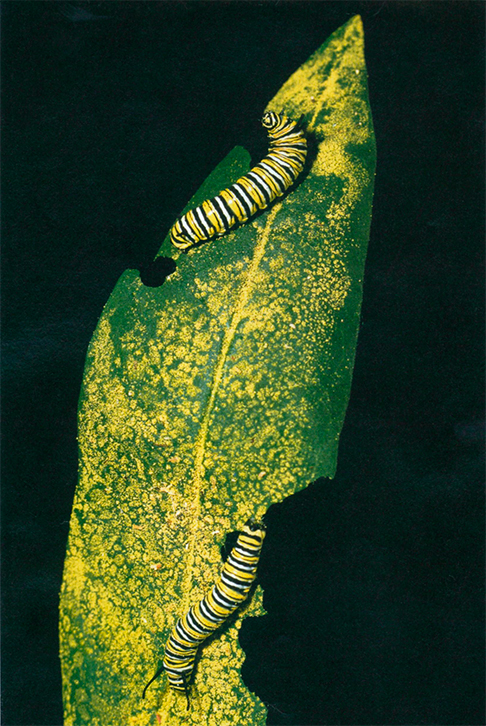
Photograph courtesy of Kent Loeffler, Cornell University.
Genetic engineering makes it possible to transfer genes from virtually any species—animal, bacteria, plant or virus—into almost any other species, no matter how unrelated the two species might be. Consequently, these revolutionary molecular techniques let scientists generate organisms with entirely new combinations of properties. For example, a jellyfish gene transferred to plants makes them luminescent, and the Monsanto Corporation is developing new varieties of grass that will produce colored lawns.
Beyond these more fantastic applications, genetically engineered crops might offer valuable benefits: increased yields, improved flavor or nutritional quality of foods and reduced pesticide use. On the other hand, transgenic crops also pose potential risks. Most public attention has focused on harmful effects to human health, including the production of novel allergens or carcinogens. But there is also a range of possible environmental impacts, including increased reliance on herbicides, the creation of new pests, harmful effects on non-target species and the disruption of ecosystem processes—concerns that have been the focus of my work. Unfortunately, scientists lack the necessary data to predict the consequences of widespread commercial planting of transgenic crops, largely because the technology itself remains so new. Nonetheless, transgenic crops are currently being planted on a commercial scale, and the area devoted to transgenic crops increased from 4.3 million acres in 1996 to 69.5 million acres in 1998. With such rapidly increasing use of transgenic crops, scientists and society must weigh whether the potential benefits outweigh the potential risks.
Scientists must ask: Do transgenic crops pose different risks from those common to crops created through traditional methods of plant breeding? After all, plant breeders used traditional methods for millennia to create organisms with quite novel traits. For example, varieties as distinct as broccoli, Brussels sprouts and cabbage came from a single species of mustard. Many scientists emphasize that the product—not the process—needs to be regulated and evaluated for risk. In other words, transgenic crops should not require regulation simply because they are genetically engineered. Instead, a transgenic crop should be regulated only if it is likely to pose elevated threats to human health or the environment. Nevertheless, genetic engineering can create many more combinations of genes and new traits than can traditional breeding. This greatly enhanced novelty diminishes anyone's ability to predict the safety of a transgenic organism on the basis of past experience.
Proponents of genetic engineering call attention to the lack of major trouble associated with transgenic crops, but that record does not guarantee that they are completely safe. As a case in point, scientists and manufacturers considered pesticides totally risk-free when first marketed in the late 1940s, and data that documented ill effects took nearly 20 years to surface. Similarly, major problems might result from transgenic crops over time. So far, few experiments have examined the safety of transgenic crops, especially the many ways that these modifications could affect the environment. Moreover, the companies that manufacture and market transgenic crops are responsible for assessing their safety, which results in a potential conflict of interest that could compromise the rigor of safety assessments. Finally, because some of the risks derive from rare, chance events—including hybridization between a crop and a weedy relative—it might take quite some time for troubles to emerge. Meanwhile, money and time for monitoring the environment after release of transgenic crops are limited, making it likely that no one would detect any sign of trouble until well after a problem developed.
To illustrate the challenges associated with assessing the risks and merits of this new technology, I shall focus on insect-resistant transgenic crops. The safety of crops engineered to be toxic or repellent to insects is a crucial concern, because such crops made up about one-quarter of the total cotton acreage and one-fifth of the total corn acreage in the United States in 1998. This rapidly increasing use of transgenic crops demands heightened rigor in testing these novel plants.
The release of organisms with novel phenotypes bears similarities to the introduction of non-native species. Many well-documented examples reveal non-native plants, including kudzu and purple loosestrife, becoming aggressive weeds with devastating environmental and economic consequences. Sometimes, introduced plants invade successfully because no insect herbivores attack them. Consequently, insect-resistant transgenic plants might be more likely to become invasive weeds than would the parental variety.

Advertisement from the June 30, 1947, issue of Time
Moreover, hybridization between a transgenic crop and a related noncrop plant can spread novel traits to additional species, which further complicates the analysis of the risk of creating new weeds. For example, Norman Ellstrand and his colleagues at the University of California at Riverside found that 12 of the world's 13 most important food crops hybridize with wild relatives in some part of their distributions. If a transgenic crop can hybridize with nearby wild relatives, the transfer of genes will be virtually inevitable once farmers plant the crop on a commercial scale. As a result, insect-resistance traits could create aggressive weeds from either the crop or, more likely, from related noncrop species. Still, some scientists argue that the escape of insect-resistance genes into wild populations would not substantially increase the population growth rate of wild plants.
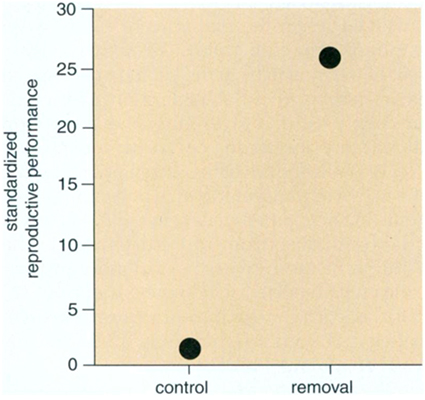
Without direct field results indicating how herbivore-resistant crops could generate weeds, some insight can be gained from ecological experiments on herbivory in natural plant populations. To quantify the impact of herbivores, scientists often manipulate the number of herbivores attacking plants and then measure changes in plant growth or reproduction. To speculate on the future impacts of transgenic crops, an investigator must summarize a large collection of such studies across many species and situations, which can be done with a statistical approach called meta-analysis. Peter Kareiva of the Marine Fisheries Service and I applied this method to results reported in 18 different publications that involved 52 different plant-herbivore combinations. We found an extraordinarily large average effect of herbivory: Plants protected against invertebrate—primarily insect—herbivores produced more reproductive structures than did 81 percent of unprotected plants. Thus any herbivore-resistance trait is likely to confer a substantial advantage, which could easily increase the occurrence of weeds. Although increases in seed production do not always translate into enhanced weediness, limitations on seed production do constrain many natural plant populations, at least in some years. Consequently, insect-resistance genes could cause nondomesticated relatives of transgenic crops to become weeds.

Marie Read Natural History Photography
The analogy between the commercial-scale planting of transgenic crops and the introduction of non-native plant species suggests several additional factors to consider when assessing the risks associated with transgenic crops. First, the vast majority of introduced plant species cause no serious environmental problems. Accordingly, most transgenic crops will probably pose little threat to the environment. As in plant introductions, though, a small percentage of transgenic varieties might become serious pests that cause vast economic and environmental damage. Second, even introduced plants that become aggressive weeds often remain relatively uncommon for long periods before becoming problematic. For example, Mimosa pigra, or catclaw mimosa, was a minor weed in Australia for about a century before it expanded dramatically and excluded other plants over large areas. In addition, my colleagues and I searched historical reconstructions of plant invasions and found many examples of remarkably long lags between the time of a plant's introduction and the detection of weed spread. Although there would be enormous pressure to discontinue monitoring efforts of transgenic crops if weeds have not been detected after many years, we must remember that detection lags can be quite long and that effective monitoring might require 30 or more years of sampling.
The second major ecological concern surrounding insect-resistant transgenic crops is that they might harm nontarget animals. For example, a plant that is toxic to Colorado potato beetles could conceivably also be toxic to nonpest beetles or to beetles that actually benefit farmers, including ladybird beetles. Two recent studies on this topic attracted considerable media attention.
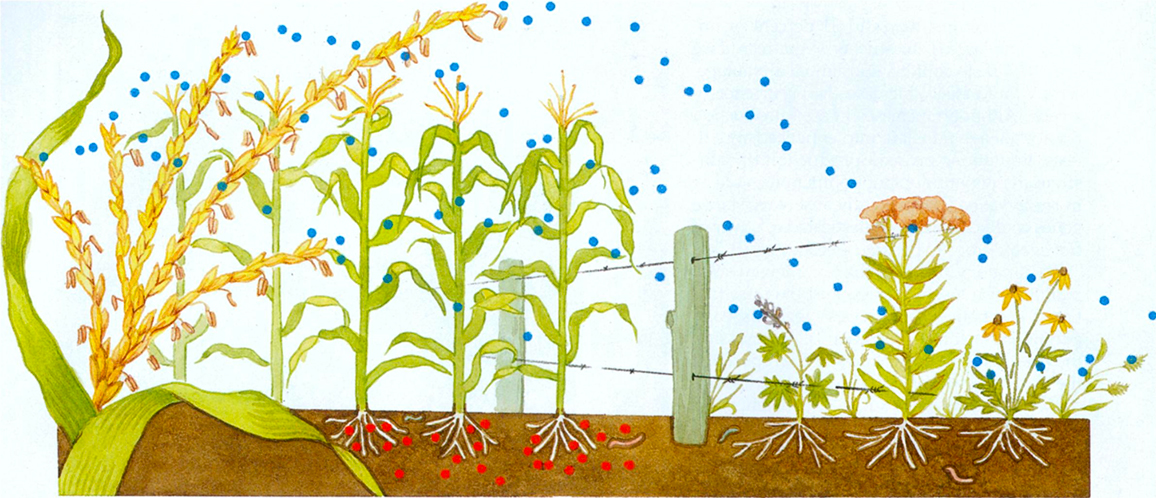
The beginning of this article mentioned one of those studies, which involved monarch butterflies. Losey and his colleagues studied the effects of pollen produced by transgenic corn that resists lepidopteran pests, including the European cornborer. Plant breeders can transfer a gene from a bacterium called Bacillus thuringiensis into corn, which causes the corn plant to produce an insecticidal compound, commonly called Bt toxin. In fact, there are several distinct Bt toxins, each capable of binding to receptors in the midgut of particular groups of insects, but humans and other vertebrates do not express these receptors. Wind pollinates corn, and its copious pollen can move up to 60 meters, coating the surfaces of neighboring noncrop plants. Thus, nontarget insects, including monarch larvae that feed on milkweed plants, consume some of the windblown corn pollen.
Losey and his colleagues coated milkweed leaves with transgenic corn pollen in quantities similar to those observed in nature. Only 56 percent of the monarch larvae survived when fed milkweed plants coated with transgenic corn pollen, whereas 100 percent of them survived when the plants were coated with nontransgenic corn pollen. Given that roughly half of the monarchs in the United States spend their summer months feeding on milkweeds in corn-producing regions, the effects on monarch populations could be substantial. Nevertheless, critics of this study point out that the toxins in transgenic corn's pollen might become inactive more quickly in the field than in a laboratory setting. In addition, C. Lydia Wraight of the University of Illinois and her colleagues reported that some varieties of so-called Bt corn express low quantities of toxin in pollen and do not significantly affect the survival of nontarget butterflies.
A second recent study, by Deepak Saxena and his colleagues at New York University, revealed that transgenic corn releases an insecticidal compound through its roots into the soil. Although Bt toxin generally becomes inactive quite quickly, the toxin can bind with soil particles and retain its insecticidal properties for 230 days or more. As a result, Bt toxin might accumulate over time and build up to much higher concentrations than previously anticipated. High levels of Bt toxin persisting in the soil could harm a variety of earth-bound organisms, which could affect rates of decomposition and nutrient cycling. Given that about 15 million acres of Bt corn were planted in the United States in 1998, scientists must quickly determine the consequences for soil ecosystems.
The findings from these studies highlight two key concerns regarding the safety of transgenic plants. First, the effects of transgenic crops will not be as localized or as transient as was initially anticipated. Rather, these studies suggest that the effects of transgenic crops might spread on the wind and persist in the soil. Second, the effects of these plants might move through food webs to species seemingly distantly associated with the transgenic crops. For both of these reasons, the ecological effects of transgenic crops will be quite difficult to predict.
Transgenic crops that produce insect toxins must undergo two separate reviews of environmental safety before they can be sold commercially in the U.S. (Approval for human consumption is a separate process.) First, the U.S. Environmental Protection Agency (EPA) reviews laboratory studies assessing a crop's effects on particular nontarget organisms, including pollinators, predatory insects and, often, soil invertebrates. Second, after collecting sufficient field data regarding a crop's performance and safety, the crop's developer may petition the U.S. Department of Agriculture (USDA) to allow commercial-scale cultivation. As of July 2000, the USDA had approved 50 petitions. Of those, 14 were for approval of crops with insect-resistance traits, all via Bt toxin. So far, the approved Bt-crop species are corn, cotton, potato and tomato.
An accurate assessment of a transgenic crop's environmental risks requires effective experimental and statistical protocols. Government organizations should label a transgenic crop as safe only if rigorous testing fails to detect any problems. Statistically speaking, a test's power is the probability that it can detect a significant difference between treatments when one exists. In practice, the statistical power of a particular test depends on the magnitude of the measured difference between the experimental groups, the amount of variability among replicates within groups and the sample size, or number of replicates per group. Of those, an investigator can most directly control only the sample size. Consequently, a rigorous test of safety must include a substantial number of replicates. Surprisingly, the vast majority of the examined toxicity studies reported in USDA petitions for deregulation relied on appallingly few replicates, usually just three or four per treatment group.
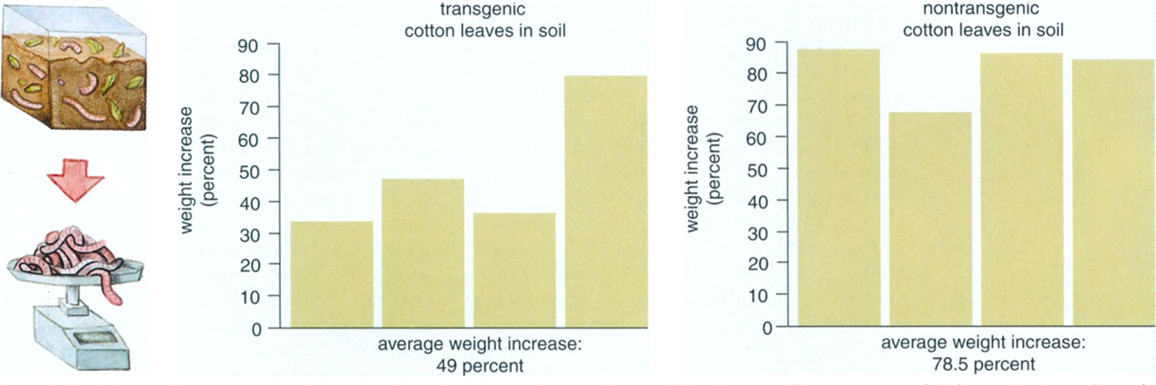
For a better understanding of assessing results, let's consider an actual example. Calgene, Inc., of Davis, California, submitted a petition (#97-012-01p) to the USDA for a variety of Bt cotton. In experiments designed to test this transgenic crop's impact on soil invertebrates, investigators placed four replicate batches of earthworms—with 10 worms per batch—in soil that included ground leaves from either transgenic or nontransgenic cotton. After 14 days, the investigators noted the weight and survival of the worms. The transgenic cotton leaves did not affect the survival of the earthworms. In fact, only one worm died out of the 80 total worms, but the 14 days represented a rather short portion of an earthworm's potential life span, which often exceeds several years. Accordingly, weight might provide a more sensitive measurement of the crop's impact. In these experiments, investigators weighed the worms in batches of 10 at the beginning and end of the 14 days. In the end, batches of earthworms that lived in the soil exposed to Bt cotton gained 29.5 percent less weight, on average, than the other earthworms. That difference, though, is not statistically significant. So, this study concluded that this particular Bt toxin did not impair weight gain in earthworms.
How believable is that conclusion? As you shall see, this study offered a very low probability of detecting a true difference between the treatments, because it relied on a small sample size (n = 4) and the data included considerable variation among replicates (pooled variance, sp2 = 273.5). In fact, if we set the probability of wrongly rejecting the null hypothesis, or a, to the standard 0.05, and we desire a 90-percent chance of detecting a true difference, or b = 0.10, we can solve for the minimum detectable difference (d) of this study:
d is > or = (2sp2/n)0.5(ta(2),v + tb(1),v)
where the t values are critical statistical values of the t distribution and v = 2 x (n - 1). Applying this equation to Calgene's experiment reveals that it could only detect a difference between treatments that exceeded 56.37 percent, which is almost twice the magnitude of the observed difference.
Moreover, the above equation can be rearranged to solve for the minimum sample size that would be needed in order to attain a particular probability of detecting a specified difference between the two groups. Suppose, again, that we desire a 90-percent chance of detecting a true effect, but now we wish to be able to detect an effect of the observed magnitude, which was 29.5 percent in Calgene's data. To do so requires only eight replicates per treatment. In other words, if the experiment were repeated with eight replicates instead of four and the variance stayed the same, a difference of 29.5 percent would be statistically significant. So, with a few additional replicates, one might have concluded that this Bt cotton did harm the tested species, and in just 14 days.
Additional evidence for worrisome experimental practices exists in a Monsanto petition (#94-257-01p) that was submitted for a Bt potato. In this case, investigators repeated experiments only when a statistically significant nontarget effect of Bt toxin was detected. If no effect was detected, the investigators did not repeat the experiment. This methodology strongly favors finding no effects even if a true effect exists. Essentially, this method resembles throwing out data—although somewhat less blatantly—unless it yields the desired answer.
In contrast to the studies submitted to support USDA petitions, some published studies—relying on larger sample sizes and more stringent methodology—documented significant impacts of Bt toxin on nontarget species. For example, Angelika Hilbeck of the Swiss Federal Research Station for Agroecology and Agriculture and her colleagues found that green lacewings, which are beneficial predatory insects, experienced 62-percent mortality when fed a diet of pests reared on Bt corn, but only 37-percent mortality when fed pests reared on nontransgenic corn. Hilbeck's group followed the fates of 200 individual green lacewings per treatment. In addition, the authors emphasized that, because Bt toxin is expressed continuously in the crop, it is essential to test for effects over the entire life span of the organisms of concern. On the other hand, Carol Pilcher, then a graduate student at Iowa State University, found no effect of Bt toxin on nontarget predators, despite using large numbers of samples.
Getting a better understanding of any potential dangers of transgenic crops, especially to untargeted organisms, will require a consistent approach to studies in this field. Currently, the EPA is developing guidelines for testing the effects of transgenic plants on collateral organisms. A Scientific Advisory Panel met in December 1999 to review the draft EPA guidelines and recommended the following: "The Agency [EPA] should consider how the data will be used and establish an acceptable level of statistical power. Based on these decisions, appropriate tests and sample sizes can be determined." (The entire report is available online at www.epa.gov/scipoly/sap/1999/index.htm.)
This panel's advice hits the target: No single prescribed sample size fits all cases. As shown above, however, one can easily calculate the necessary sample size after collecting some pilot data. Although it is popular these days to complain about federal regulations and interference, the EPA's recognition of the importance of statistical power makes clear the benefits of governmental guidelines. If the EPA does not step forward and set standards for experimental design, some companies would likely continue to rely on extremely weak tests of nontarget toxicity.
The public knows that some past technologies—including DDT, PCBs and others—caused major environmental problems, despite repeated assurances of safety from scientists. Undoubtedly, that history contributed to the public's current concern over transgenic crops. Those who stand to profit from transgenic technologies view this heightened public vigilance as unfounded, even hysterical, and caution that increased regulatory oversight would hinder progress. Nevertheless, the public stands to lose if transgenic crops cause damage to the environment.
Risk analysis should reveal how the public good might suffer if new technologies backfire. Nevertheless, transgenic crops offer some special challenges in applying ecological-risk analysis. Currently, investigators evaluate risks by analogy or by direct experiments. Analogies to exotic, invasive plants fall short, because transgenic crops are not entirely new to an area, but rather are modifications involving only a few traits. Analogies to familiar crops also fail, because the mingling of genes in transgenic crops can produce completely novel traits. This leaves direct experimentation and monitoring as the primary tools for risk assessment. To be completely assured of the ecological safety of a transgenic crop, however, would require many experiments: testing the transgenic plant in different environmental conditions, at different times of year, in combination with different farming practices, and examining the effects of the plants and plant by-products on an enormous number of species that could potentially be affected by the transgenic traits. Clearly, attaining this level of certainty is neither reasonable nor possible.
Consequently, we must decide how we will deal with the unavoidable uncertainty that accompanies transgenic crops. In essence, current regulations assume that a transgenic crop is safe unless it is shown otherwise. Alternatively, we could assume that a transgenic product is unsafe until the manufacturer demonstrates its safety. Despite recent studies that highlight possible risks, plants engineered to express Bt toxin are almost certainly safer than most chemical pesticides, which generate well-established dangers for nontarget arthropods. Nevertheless, an extraordinary variety of novel traits will be incorporated into tomorrow's transgenic plants, and the safety of those plants will vary substantially. If the assumption remains "safe until proven otherwise," regulators must boost the rigor of testing. Experiments with a handful of replicates and low statistical power could fail to expose the environmental risks associated with particular transgenic varieties.
The author thanks Kay Peterson of the USDA for her rapid response to requests for copies of petitions and Michael McKee of Monsanto for providing information about nontarget studies. In addition, the author thanks Peter Kareiva and Polly Goldman for comments on an earlier draft.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.