For a Few Atoms More
By Roald Hoffmann
When the game becomes less of a game
When the game becomes less of a game

DOI: 10.1511/2008.70.104
To enhance our appearance we will do terrible things to our bodies. And when there is money—or its correlate, fame—to be gained, athletes will seek to enhance their performance in sometimes terrible ways, using chemicals, natural and synthetic, to make themselves stronger, faster, leaner. With consequences that may be terrible.

Tim de Waele/Corbis
This has probably been true for millennia. I recently passed pedestals hailing the athletes of ancient Ephesus, now in Turkey; I am sure they tried diets and herbs to get their statues on those pedestals. It's not just professional athletes who are responsible: Nations (such as the former German Democratic Republic), and we ourselves share the blame, with our gladiatorial instincts and (male dominated?) dependence on the forces of fandom and partisanship.
In the recently released "Report to the Commissioner of Baseball of an Independent Investigation into the Illegal Use of Steroids and Other Performance Enhancing Substances by Players in Major League Baseball," former Senator George J. Mitchell says, "For more than a decade, there has been widespread anabolic steroid use," and "the illegal use of anabolic steroids, human growth hormone, and similar drugs poses a serious threat to the integrity of the game of baseball." Barry Bonds has been indicted for perjury and obstruction of justice in connection with his testimony denying anabolic steroid use; his trainer has been convicted of distributing steroids. Marion Jones has admitted to lying about her use of a steroid before the Olympics in which she won five gold medals.
What is going on? How and why did our athletes come to use "the clear" and "the cream," as Bonds and Jones called the substances their trainers gave them? What are these substances? And how do we detect them? In an approach to this sordid story, in which no one comes out clean, let us go back to the sport regrettably tied most closely to doping in the public imagination, competitive cycling.
The 2006 Tour de France winner, Floyd Landis, was reported to have failed a testosterone drug test. More of what was actually found in his urine in just a while. Race officials collected a sample after his comeback victory in a critical stage of bicycling's premier race. A second sample confirmed the problem, and eventually Landis's victory was disallowed. Appeals followed; as the case stands now, Landis has appealed to the Court of Arbitration for Sport to overturn the decision against him.
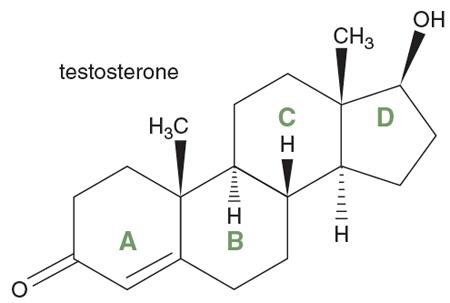
Testosterone is the principal male sex hormone, produced mainly where you would expect from its name. It is also made in the ovaries of females. Testosterone is an anabolic compound, so-called because it promotes the growth of tissues such as muscle and bone; testosterone is also a steroid, member of a class of molecules that gives us a continuing lesson that almost the same is not the same.
All the steroids have the same atomic framework—four all-carbon rings, fused together. Three are hexagons, the third ring going off at an angle to the other two. Fused to that last ring is a pentagon of carbon atoms. Call the rings A (6 carbons), B (6), C (6) and D (5). Testosterone has an oxygen and a hydrogen (OH) attached to ring D and two CH3 (methyl) groups, one at the juncture of rings C and D, the other at the juncture of A and B. Ring A contains a double bond and has an oxygen attached to it as well.
Testosterone is responsible for the secondary sex changes that occur in male puberty—facial and pubic hair, oily skin, body odor, all that teenage-boy stuff. But the molecule is also produced by human females, albeit in one twentieth of the amount in males. In both sexes, testosterone affects energy levels and protects against osteoporosis. Nothing is simple in the real world—only human beings want it black or white, male or female.
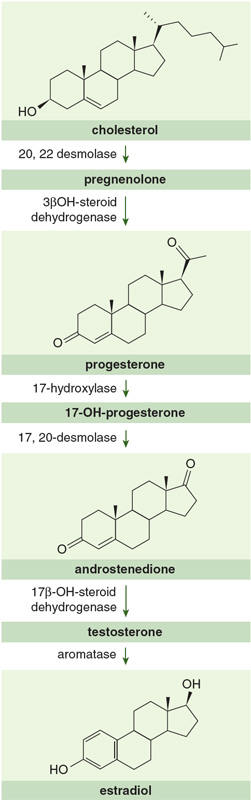
Remarkably enough, the biochemical precursor of testosterone in both sexes is progesterone, a female sex hormone. The difference between progesterone and testosterone is all of four atoms—two carbons and two hydrogens—on the five-membered D ring. Two other female sex hormones, estradiol and estrone, differ from testosterone by the loss of CH3 and an H from the former plus two more hydrogens from the latter. Small changes, indeed, but ones with major consequences.
Stephanie Freese
Other molecular family members with the same 6:6:6:5 fused-ring pattern include ecdysone, the molting hormone of insects; cholesterol, an essential, abundant part of our bodies; cortisones, which are important anti-inflammatory drugs; and bile acids. A pretty incredible set of biological functions, n'est-ce pas? All made distinctive with one less atom here, one more atom there.
It's fun to figure out this exquisite biological diversity, but why should a biker take testosterone? And how did the testers find out that Landis did?
Testing for abuse is not simple. Blood concentrations of testosterone vary widely between individuals and within one individual over time. So one cannot conclude from just an elevated level of testosterone that the molecule has been supplemented!
Stephanie Freese
Enter epitestosterone, a stereoisomer of testosterone. In other words, it contains all the same atoms as testosterone, attached to each other in similar ways, but with a different disposition in space. In particular, the OH group of epitestosterone points "down" in the picture shown, instead of "up" toward us, as it does in testosterone. It turns out that epitestosterone has no apparent physiological effect (the same and not the same redux). Both testosterone and epitestosterone are produced in the body in similar amounts, by distinct biochemical pathways. So whereas there may be a higher absolute concentration of testosterone (and epitestosterone) in one person compared with another, the ratio of testosterone to epitestosterone is close to 1 for both of them.
This is the clue to detecting abuse. Supplementing testosterone, the only isomer that has the desired physiological effects, doesn't change the biological production of epitestosterone. So the sports-medical bodies settle on the testosterone:epitestosterone ratio as an indicator of foul play. Ideally, one should have a profile of that ratio for every individual. In the absence of this profile, one makes liberal assumptions for the entire population: near 1:1 is normal, 4:1 is when the red card is shown. Landis's samples apparently had an 11:1 ratio.
I know, I know—you will tell me that the dopers, making big bucks, are not stupid. They'll give not only testosterone, but also some epitestosterone, so as to keep the ratio of testosterone:epitestosterone under 4:1. The sports "doctors" in the GDR did this 25 years ago.
With good science, this strategy too can be countered. Natural testosterone differs from the synthetic material in its ratio of carbon-12 to carbon-13 isotopes. The synthetic molecules are made from precursors derived from steroids found in certain plant oils, such as soybean. These plants biosynthesize their steroids from smaller building blocks of three carbons, somewhat different from those found in many of the plants we consume (and which then go into our testosterone), which form C4 molecules. A consequence of the different biochemical mechanisms is an isotope effect, a slight difference in the way 12C and 13C (present naturally in small amounts) are incorporated. The net result is that our testosterone is ever so slightly (and detectably) richer in 13C than synthetically derived steroids.
What is puzzling in this story is why any bicyclist would take testosterone on one isolated occasion (Landis was tested at other stages of the race, and nothing showed up in those samples). Anabolic, muscle-mass-building processes require the prolonged use of testosterone. Perhaps its use was an act of desperation by a superb cyclist who was behind. The irony is that it may have functioned not because of what it was, but as a placebo.
The Bay Area Laboratory Cooperative (BALCO) had a good business going. It provided athletes and their trainers with a variety of performance-enhancing substances. Several BALCO principals have been convicted of various crimes connected with distributing, among other things, anabolic steroids. Two of these substances are of particular interest—"the clear" and "the cream." Barry Bonds received both from his trainer Greg Anderson; Bonds has said he was told the former was flaxseed oil, the latter a rubbing balm for arthritis. Marion Jones said similar things about the substances she got from her trainer, Trevor Graham. Jones said she noticed changes in her body after she stopped using the products and admits "Red flags should have been raised in my head when he [Graham] told me not to tell anyone. . . ." Bonds apparently noticed nothing.
Stephanie Freese
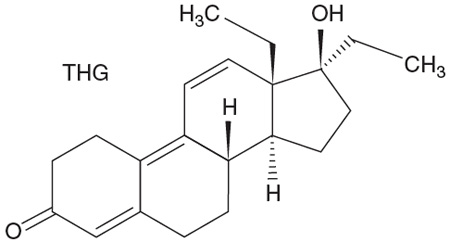
What are these substances, which BALCO principal Victor Conte obtained from chemist Patrick Arnold (more on Arnold below)? Well, it turns out that "the clear" is a solution of a steroid, tetrahydrogestrinone (THG). The skeleton should look familiar. The drug was banned internationally in 2003, but not until a trainer (the above-mentioned Trevor Graham; that's another story) sent a syringe of the stuff to the United States Anti-Doping Agency. Prior to that, no one tested for it—because they didn't know it existed. And "the cream"? That turns out to be mainly our old friend, a mixture of testosterone and epitestosterone, in a ratio that will not trigger an alarm.
Some of the rotten apples in this story are chemists, my own clan. Patrick Arnold pleaded guilty to a count of selling controlled substances (he actually supplied much of the BALCO material). A pro-steroid website lauds this "father of prohormones" as "a chemist who is responsible for the introduction of androstenedione [another anabolic compound] to the market as well as other second and third generation prohormone products." It continues, "Always supporting the industry, Arnold is also the President of the Prohormone Research Organization (PRO), a lobby group assembled from some of the most influential members in the supplement industry as well as the antiaging community. PRO is committed to providing legislators and government officials with truthful, scientific information about prohormones and other dietary supplements." The scientific information that exists (and much work needs to be done) points to long-range biological harm in nonmedical, excessive use of anabolic steroids. But good science is not what Arnold had in mind. Arnold "cut his Ph.D. studies short to pursue his own business venture."
THG is a "designer steroid." With so many sites for substitution on that skeleton of four fused rings, there are many ways to change this structure—medicinal chemists have been doing it for years. Many of the resulting molecules will have no physiological effect at all (remember epitestosterone?), others will be poisonous, and still others will prove to be anabolic and really harmful in large doses.
How harmful? The problem is that these drugs have seldom been studied in detail (excepting testosterone, of course). When used illegally by athletes, there is no standard dosage. Anecdotal evidence indicates that athletes are reaching steroid concentrations 5 to 30 times greater than the natural level of testosterone in the body. The list of potential effects begins with acne, hirsutism, changes in body shape and voice, and increased sebaceous gland activity. The list goes on to include permanent muscle fiber damage, breast enlargement in men, breast diminution in women, effects on sexual organs, and liver damage.
Asking the question "Why would anyone risk doing that to themselves?" ignores human nature and shifts the blame away from ourselves. Our gladiatorial (spectator!) instincts and our own active glorification of athletic prowess are important parts of what makes young people do such foolish things.
It is relatively easy to make new steroids and test, in a rough way, which are anabolic. An average chemist (as you see, no Ph.D. needed) can do it. The chemistry, like that involved in the transformation of cold-medicine pseudoephedrine to street-drug methamphetamine (another dismal story), is really simple. The making of some steroids may require skillful hands. But they too can be hired.
So is this a losing battle? To the extent that we are struggling against ourselves, to the extent that our clamor for sports victory perversely encourages the formation of muscle at any cost (voilà! 300-pound football players in high school), it's hard to think that anything will change. A few will maim themselves for the dream of money or fame. The market to supply them, to think up ever more ingenious ways of subverting the doping tests, will not disappear. And chemists somewhere will do the dirty work. Of course, the institutions we create, that one might think would control unfair and illegal use, are no better. The reaction of the executive director of the Major League Baseball Players Association to the Mitchell report was shamefully evasive and legalistic.
The hope is that there is a strong place in the human dream for a level playing field. And a special feeling for the disastrous effect steroid use can have on children, whose aspirations are focused on athlete-heroes and heroines. The national and international anti-doping agencies can also hire good chemists, and develop tests for potential new anabolic steroids. We can relearn to see the action in our softball teams, instead of the "major" leagues (for a kid who lived on Bedford Avenue, the world ended anyway when the Dodgers left Ebbets Field). And I will keep on cycling, on my own Tour de Ithaca.
© Roald Hoffman
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.