Thermophiles in Kamchatka
By Roald Hoffmann
Strange life in Russian hydrothermal pools
Strange life in Russian hydrothermal pools

DOI: 10.1511/2001.14.20
When Stefan Petrovich Krasheninnikov, a 24-year-old Russian student of science, first visited Kamchatka in 1737, he saw mountains that "for many years throw out a continual smoke, but flame only at times." He wrote, "the principal riches of Kamchatka consist in the great number of wild beasts: Among them are foxes, sables, stone foxes, hares, marmots, ermines, weasels, wolves, reindeer, wild and tame, and stone rams." Clearly his mind was on the furs precious to Russians and Chinese. That's why Empress Anne had sent him (actually she had sent some professors of the Russian Academy of Sciences, who opted to stay behind in Siberia and send their student on into the wild lands); this was the reason bands of unruly and rebellious Cossacks were dispatched to subdue the land, exacting tribute in furs from natives.
It did not take me four months to reach Kamchatka, as it did Krasheninnikov from nearby far-eastern Siberia. Only four hours on an aging jet of Reeve Aleutian Airlines from Anchorage. Distant from the Czarist and Soviet sources of power, this was never a rich part of Russia. It's a more than nine-hour flight from Moscow to Petropavlovsk-Kamchatsky, situated on a beautiful natural harbor, which became (along with Vladivostok) a seat of Soviet military power and its main eastern submarine base. The harbor is filled with rusting, decomposing ships.
Like Krasheninnikov, I could not help seeing the volcanoes—it is hard to look up anywhere in Kamchatka and not see one in the landscape of the most seismically active place in the Pacific Rim of Fire. And I came to see life. Not fur-bearing mammals, but a microscopic, far older form of life, the thermophiles and extremophiles of the hydrothermal sources of Kamchatka.
Workhorse Russian troop-carrier helicopters flew us into the Uzon Caldera. Two hundred thousand years ago a volcano erupted here. The frequent earthquakes fragmented the deposits in the caldera. But around was hard rock, sealing in a kind of giant chemical reactor. Several kilometers below the surface, a magma chamber is buried. Surface water seeps down to it, is sent up as hot steam, corrosive, mixed with volcanic gases. There's dissolution and redeposition of ions under conditions of varying acidity and lots of time—it's a veritable hotbed of geochemistry. Beneath the surface lie layers rich in arsenic, phosphorus, copper, lead, antimony, even gold. Hydrogen sulfide (H2S) and sulfur are never far away. We saw a little pool, a meter across, bubbling merrily away at 95 degrees Celsius and depositing at the surface a beautiful yellow-orange layer of As2S3.
From far away, Uzon is nondescript. Thin "smoke" is the first thing one notices, rising from some shallow lakes. Closer up the lakes and surrounding hills seem a Monet-like landscape of blues, grays, greens and yellows blending into each other. The smoke turns out to be condensing steam; it does the blending. Krasheninnikov saw those marvelous colors too, in the microcosm of a rock; he wrote "the clay in taste is sour and astringent; and if a piece of it, or a stone, is broken, there appears an efflorescence of alum, like a moss, with the colors blue, white, red, yellow, green, and black, which are so mixed as to resemble marble; and when the day is not quite dry, the colors are pretty bright."
Still closer, the pastel landscape breaks up into pools. Some are crystal clear, some muddy, filled with clay. Except suddenly a bubble pops in the clay, more burst explosively, and soon, especially as we smell the hydrogen sulfide, it looks like a nook reserved for some of Dante's less favorite people. The subtle color around the pools comes from mats of bacteria and Archaea. Sometimes we see several rings of slightly different color, each a species flourishing in a different temperature range.
Our group is an international one, nearly all microbiologists here for a small workshop on the enzymology, molecular biology and biochemistry of thermophiles such as live in these springs. A graduate student in the group sticks in a probe with a thermometer, another a piece of pH paper. The water is bubbling up at 95 degrees Celsius. The pH is 1, about 0.1 molar of sulfuric acid. I would not immerse my finger in that water even if it were cool. Two weeks later, far away, a Yellowstone Park employee dies in an accident, stumbling into a similar boiling pool.

Roald Hoffman
Now I know what a thermophile likes. The world's record is an organism called Pyrolobus fumarii that flourishes at 113 degrees Celsius (under pressure, at sea bottom). The pools in Kamchatka are not only acidic, but also basic, up to pH 10.5. When I see life rampant under such conditions, or plants growing in what was lava weeks after an eruption, I can't help thinking that the variegated surface of Mars once bore life too.
In the course of the week, I learn a lot about thermophiles. They are some bacteria and mostly Archaea, the third kingdom of life recognized only in recent decades. Most are anaerobic, not requiring (and sometimes damaged by) oxygen. They are the same and not the same. Of course they have membranes and proteins and nucleic acids, all the wondrous molecular machines of the living. But a normal lipid cell wall would decompose at these temperatures, the hydrogen-bonded helices of proteins would unwind, the genes would fragment and not be faithfully reproduced. An acid and hot environment is what people normally use to denature proteins; these creatures love it.
So they are different. Their lipids are linked by sturdier ether groupings instead of esters. Thus bonded, lipid chains span the entire membrane of the cell to form a monolayer wall. The proteins of thermophiles appear to be reinforced by special sequences of amino acids, and have increased ion-pair content buried inside the protein, which, it has been argued, leads to greater intrinsic stability. A better pH environment is maintained inside the cell. Auxiliary cell components, such as polyamines and small basic proteins that resemble histones in eukaryotic chromosomes, seem to stabilize nucleic acids.
I've never seen salmon prepared in as many tasty ways as at the rural hostel where we stayed. Thermophiles have to eat too. The building blocks of the remarkably varied archaeal diet in the hydrothermal fields (or submarine volcanic vents) are CO2, CO, H2, H2S, N2, S and several oxidized sulfur species. That more than suffices, in the presence or absence of oxygen. Some typical reactions are
S + 3/2O2 + H2O —> H2SO4
H2S + 2O2 —> H2SO4
CO2 + 4H2 —> CH4 + 2H2O
Normal green-plant photosynthesis is endothermic, by 481 kilojoules (change in free energy per mole CO2). The energy to get it done comes from light. Who needs light when you can have the H2S reaction above? It releases 706 kilojoules per mole! That's enough energy to reduce lots and lots of CO2 to sugar.
Most remarkable are biological systems that use as their source of energy inorganic ions such as Fe++ and Mn++. Thiobacillus ferroxidan's name tells it all. The oxidation of such metal ions is exothermic, and of course it is used. It is conceivable that much magnetite is of biological origin.
I didn't expect to face questions of intellectual property rights and ethics in Kamchatka, but.... As described above, the molecular machinery of thermophiles works—in ways we don't yet completely understand. It does so under conditions that approximate more a heated reaction flask in a lab or a washing machine than an Ithaca winter. This has not escaped the attention of detergent concocters, who want an organic stain-removing enzyme, as well as biochemical supply houses. One enzyme, Taq polymerase, which is used in the polymerase chain reaction, apparently has a billion-dollar market.
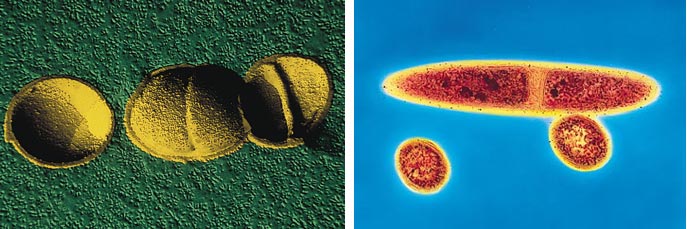
Scimart / Photo Researchers, Inc.; Kari Lounatmaa / Science Photo Library
Taq polymerase was isolated from a thermophilic bacterium, Thermus aquaticus, collected in Yellowstone National Park. The park receives not a penny from the manufacturers. The consequences: People get ideas—maybe there are other Taq polymerases around. Maybe we can remove dioxins, make a carbon-carbon bond in another, more efficient way. There's money to be made in bioprospecting. There were bioprospectors among us in Kamchatka. And not all working for companies. One industrial biologist was reported as saying, "I've never met an academic who didn't have something to sell." Or who wasn't thinking about a small startup company.
Second, you can understand that Yellowstone National Park is not happy about not getting anything back. How to reward, legitimately, a source of materials that will yield commercial profit—a place, a country? Licenses for sample collecting at Yellowstone now carry a proviso for a royalty if a product from an organism collected in the park is commercialized. I have a feeling that in the absence of a clear agreement of this kind, it will take many a court case to decide what happens if a bioprospecting company takes the DNA of a natural organism, and mutates it in the lab or splices it with a piece of DNA from another organism so as to enhance the production of an enzyme. Whose DNA is it at the end?
This was the first time I was in a meeting of biologists. I noted the following things:
(1) The more molecular they were, the less I understood. The reason was that I have been bypassed by several generations of biochemistry, molecular biology and genetic engineering. And what jargon! It was my fault to let it happen, but also there were a couple of people there who could not conceive that someone could not know what 16S rRNA was. (I learned eventually that it is part of the ribosome. By comparing the sequence of 16S rRNAs from various organisms, scientists can make rough determinations about their evolutionary relatedness.)

Photograph courtesy of Roald Hoffman.
(2) I imagined that in the struggle between the organismic and the molecular, the molecular worldview had routed the opposition. Indeed, I saw signs of the struggle. A preeminent expert on thermophiles, Karl Stetter, a man responsible for naming a good fraction of the archaea, used some of the language of the DNA world, but clearly wanted to point to its limitations. So he stressed that two species close on the 16S rRNA scale—and therefore presumed to be close on the evolutionary tree—nevertheless had very different metabolisms. I also saw people who were in a way uninterested in the organisms, in the beauty and complexity of their adaptations to the hostile (to humans) environment. Instead of looking under a microscope, they were just eager to get the DNA out of the bugs.
On balance, what was interesting to me was a new breed of microbiologist—delighted to collect the Kamchatka microfauna and eager to see its DNA. It was wonderful to see the enthusiasm of Stetter fishing up a sample (you can be sure it was not his first one) from a boiling well and to see young biologists using the hotel lobby floor as a makeshift chemical laboratory to fix the samples they had collected earlier in the day.
(3) I saw a new ecology at work: Eric Mathur of Diversa Corporation, as he dipped his collecting receptacle under a tree stump near a hot spring and noted with excitement how probably no one had looked in that environment. Maybe there was a group of organisms there, he continued, to oxidize xylenes. Maybe a gang of enzymes. Whether by looking at the organisms, or by looking at their DNA, the interest of the microbiologists was in an ensemble, not the individual. People were clearly thinking in terms of populations.
I bought a small piece of walrus scrimshaw in Kamchatka. In exquisite brown lines, it shows a most Russian well-dressed bear sitting on a sled in the snow. He's tired but happy, perhaps after a long hunting trip. That's how my microbiologist friends looked after a long, wet day of collecting. The bear holds cupped in his hands a steaming cup; there is a teakettle sitting in the snow. So he's a thermophile too. And behind him is a smoking volcano. It's Kamchatka.
© Roald Hoffmann
Thanks to Valeria Rozenberg and Sergei Varfolomeyev for getting me to Kamchatka, as well as to Frank Robb and John Varley for their comments.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.