
This Article From Issue
January-February 2019
Volume 107, Number 1
Page 10
Alexandrite is a rare form of the mineral chrysoberyl, which has the chemical formula Al2BeO4. Not content with being one color, it can display a whole range of hues, depending on the light falling on it. Deposits of alexandrite were first discovered in the 1830s, in the Ural Mountains in Russia. Mineralogist Count Lev Alekseevich Perovskii presented it to the future Tsar Alexander II on his 16th birthday and named it in his honor.
Alexandrite’s color is a consequence of impurities present in its chemical structure. In some places where an aluminum ion should sit, a chromium ion can be found instead. These impurities account for less than 1 percent of the aluminum sites, but this amount is enough to give alexandrite its hues.
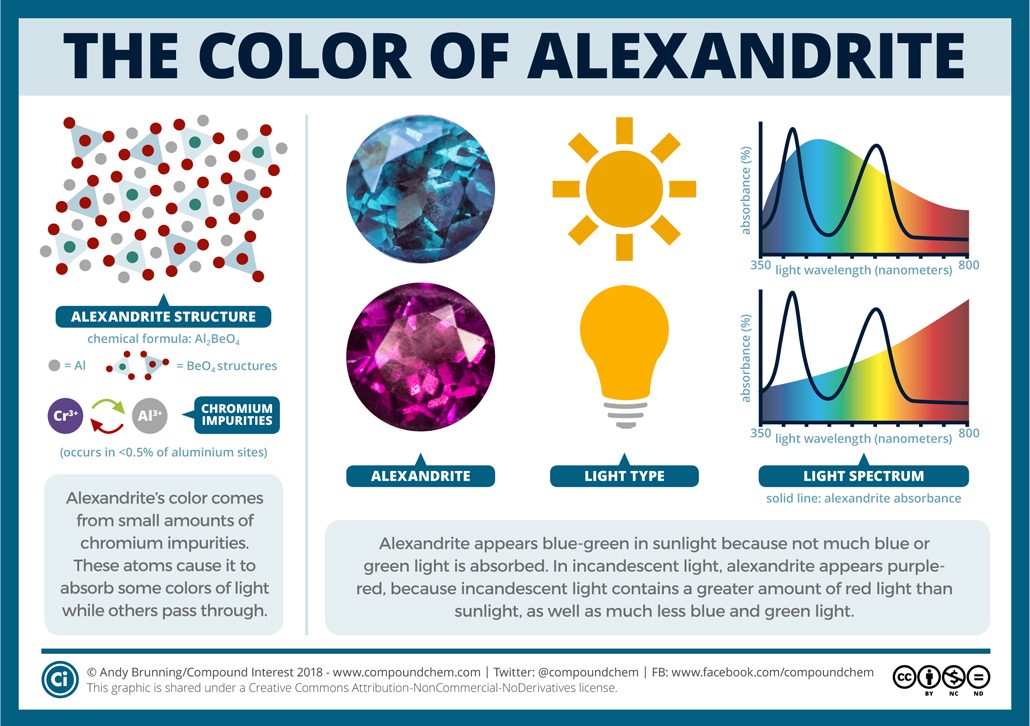
Chromium ions absorb visible light strongly in the dark blue and yellow regions of the spectrum. Sunlight doesn’t have a uniform contribution from all colors in the spectrum; there’s slightly more green and blue light than red. Because more green and blue light remain unabsorbed than red light, alexandrite appears blue-green. In incandescent light, candlelight, or any light that is what we’d call “warm,” the red end of the spectrum makes a much greater contribution. There’s also much less blue and green. Because chromium ions don’t absorb much red light, this leads to the purple-red coloration of alexandrites in these conditions.
It’s even possible to get one more color from alexandrite. If an ultraviolet lamp is shone on an alexandrite, an intense, glowing red color is seen. The electrons in the chromium ions absorb the ultraviolet light, gaining energy and jumping to higher energy levels. As they fall back down to their original energy level, they emit their excess energy as light.
Alexandrite is rare and pricey, but some synthetic versions are close to the naturally occurring ones. Good synthetics mimic the original gemstone’s color-changing abilities more faithfully. Two methods are used to produce synthetic alexandrites: the pulled crystal (or Czochralski) method and the flux-melt method.
In the pulled crystal method, gemstone constituents are melted in a crucible. Then a small seed crystal is lowered into the melt. As the seed crystal is slowly raised from the molten solution, it increases in size as constituents from the solution crystallize onto it. Alexandrites produced with this method have intense colors and are nearly flawless.
The flux-melt method uses a molten flux, or solvent, of variable composition, in which the alexandrite constituents are dissolved. The dissolved components can attach to a seed crystal placed within the flux, or crystallize randomly if no seed crystal is present. The process can take up to 12 months, with the slow cooling more adequately mimicking the natural processes that form alexandrite. Small amounts of flux can be trapped in the alexandrite crystals that form. But these impurities make synthetic gems harder to distinguish, because they appear similar to inclusions in real alexandrite.—Andy Brunning

American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.