Curbing Immune Cells’ Appetite
By Robert Frederick
Infected cells produce an “eat me” signal so that they’re destroyed by the immune system. But what if you want an infection to last?
Infected cells produce an “eat me” signal so that they’re destroyed by the immune system. But what if you want an infection to last?

Few people want a viral infection of their brain tissue or spinal cord. The viruses that naturally infect the central nervous system include Zika, HIV, rabies, and polio. But researchers have been using other viruses to manipulate the genomes of cells in the brain and spinal cord with the goal of better understanding how the central nervous system works, including how it defends itself. Clinicians have even applied some of their research to treating people, delivering gene therapy via viruses to correct for genetic deficiencies.
The immune system has evolved to combat viral infections, though, even if viruses are engineered to have a positive effect. “The immune system is also clever and has ways to recognize any kind of perturbation—it may not necessarily recognize the virus itself, but will recognize the perturbation that the virus introduces,” says Axel Nimmerjahn of the Salk Institute. In addition to removing the virus or altered cells, the immune system may overreact, Nimmerjahn says, destroying not only infected cells but nearby cells, too. That can lead to lifelong cognitive or motor impairments, because few cells in the central nervous system regenerate.
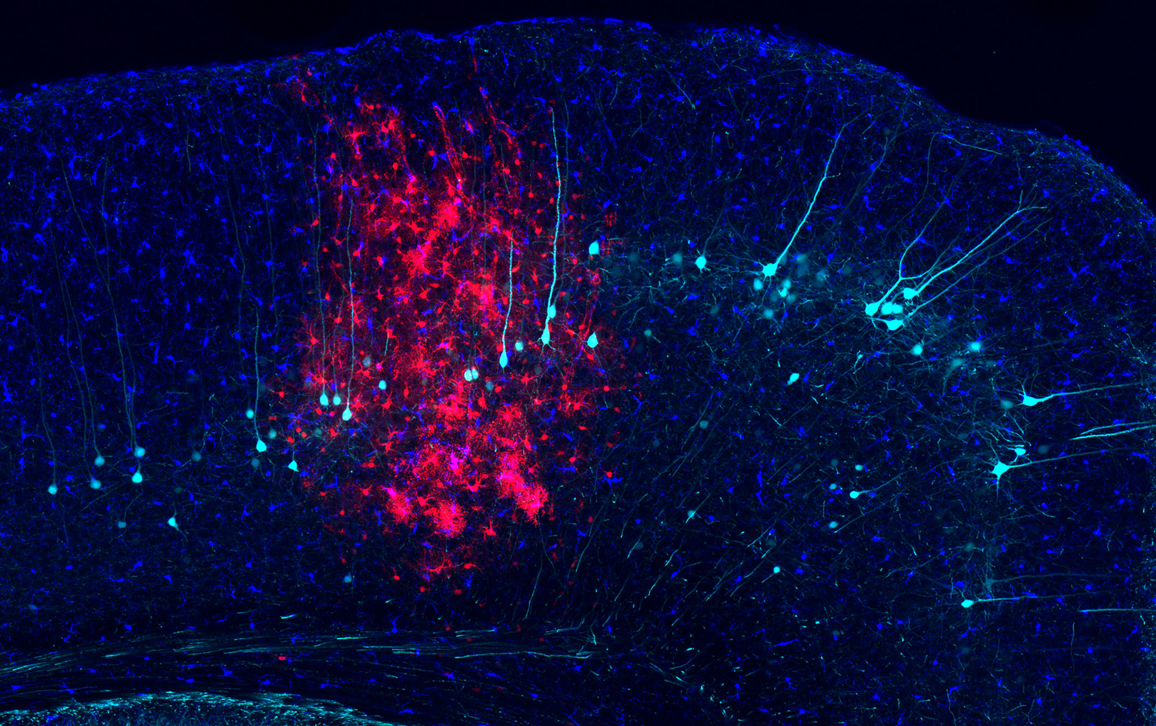
Nimmerjahn et al.
One way to prevent that outcome is to dampen the “eat me” signal identified by Nimmerjahn and his team. This eat-me signal is produced by infected central nervous system cells and tells the immune system’s microglia cells (shown above in dark blue) to attack. The researchers discovered that inhibiting the activity of a key protein, phospholipid scramblase 1, prevents that signal and protects virus-infected cells (astrocytes, shown above in red) from being eaten by the microglia for at least six months, which is how long the team measured the effect.
“If you didn’t do anything,” says Nimmerjahn, “many of these infected cells would be removed by microglia within days to weeks.” The team published the results in the February 8 edition of the journal Neuron.
Five years ago, the team had been primarily investigating the function of astrocytes, which are thought to regulate much of the activity within the central nervous system. To manipulate the function of these cells, the team screened many viruses and found adenoviruses to be particularly potent. But it became clear—as it had to other researchers who introduced adenoviruses into the central nervous system—that the immune system’s overreaction to adenoviruses presented a challenge to their research on astrocytes.
“We actually hadn’t done this research in the context of gene therapy,” says Nimmerjahn, “but of course, now that we have a way of controlling the immune response, that is something we’re now exploring.”
Adenoviruses, causing illnesses such as the common cold, were some of the first viruses used in gene therapy trials because of their potency. But they fell out of favor because of the pronounced immune response they prompted. Now, having discovered a path to overcome that response in the central nervous system, Nimmerjahn plans to make use of the advantages adenoviruses have over other viruses in continuing his team’s research on astrocytes. In addition, the researchers are concurrently searching for a small-molecule drug to do the same thing, which would also allow for the treatment of other infections that activate phospholipid scramblase 1.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.