Hungry Little Beasts
By Robert Frederick
A low-emission method of combustion is full of puzzles and potential.
A low-emission method of combustion is full of puzzles and potential.

DOI: 10.1511/2016.123.332
With the power of both tornadoes and fire, few things are more destructive than a fire whirl. Even in the safe, controlled environment of the lab, fire whirls can cause mishaps: “We burned down our equipment and were set back six months in the experiments,” says Elaine Oran about the one—and only one—time her colleagues and students added a little too much fuel to their fire-whirl experiment.
Images courtesy of Huahua Xiao, Michael J. Gollner, and Elaine S. Oran.
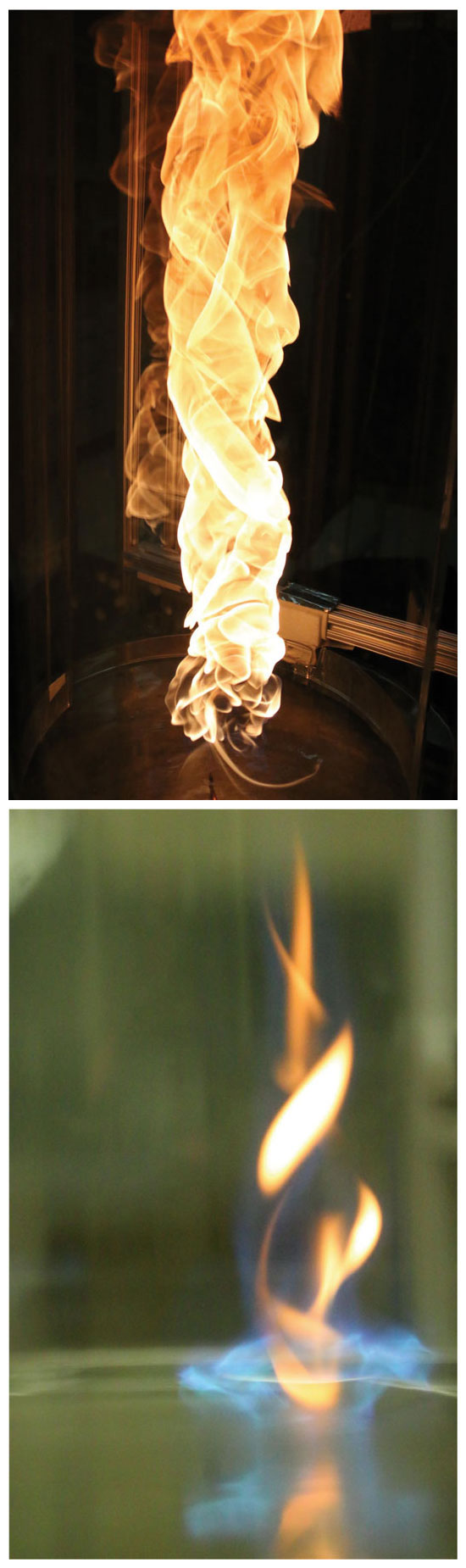
Along with her colleagues Michael Gollner and Huahua Xiao at the University of Maryland, Oran was experimenting with fire whirls after seeing a video of one on water. The video showed the aftermath of a lightning strike in Kentucky on a warehouse full of Jim Beam bourbon, a lot of which had spilled into a nearby lake and caught fire. “It must have smelled wonderful,” Oran says. At first, the alcohol burned as a pool fire, without any rotation. Then a rare fire whirl formed. Oran noticed how it was drawing all the fuel from the surface of the lake to it, becoming increasingly intense.
That intensity made it different from fire whirls in forest and brush fires, which tend to produce black, sooty smoke. More videos confirmed that fire whirls burning liquid fuel on water produce more white smoke as they grow more intense, indicating less soot and higher efficiency.
So the team started experimenting with small-scale fire whirls over water. Their pool of water is just a few centimeters deep and 40 centimeters in diameter. With slightly offset quartz half cylinders to both enclose the fire and allow air to flow in along their entire height, the team squirted a bit of n-heptane (a component of gasoline) onto the water, lit it with a butane lighter, and stepped back.
Experimenters sometimes use fans to create a vortex and then add fuel and fire to the mix. “If the geometry is correct to create the initial vortex,” Oran says, “you don’t need to add rotation.” Sure enough, shortly after the pool fire began, a fire whirl formed (right, top). But after only a few seconds, the fire whirl evolved into a small blue whirl (below)—something no one had ever seen before.
Varying the setup and using square enclosing walls instead of the two half cylinders didn’t make a difference in the fire-whirl formation; neither did alternate fuels. Burning crude oil produced the blue whirl, which excited the students who were running that particular experiment. They decided to add a bit more oil. It turned out to be a bit too much. Still, once they had safely put out the fire, the results suggested that blue whirls might one day be used to help clean up oil spills if, after blue whirls are well understood, researchers also understand how to deploy them safely.

Images courtesy of Huahua Xiao, Michael J. Gollner, and Elaine S. Oran.
That will take time. Although combustion experiments are relatively easy, Oran says, “what’s expensive is the diagnostics.” The temperature inside blue whirls is still unknown, but Oran speculates it to be around 2,000 degrees Celsius. No one yet understands the structure and shape of the blue whirl, whether it can be generated directly or must first be a fire whirl, or if it forms at larger scales. “And that’s why I feel that a [computer] simulation here would be so important,” Oran says, “because it would allow us to jump a few steps.”
So there’s still a lot of physics to explore in understanding the blue whirl, “a little hungry beast,” as Oran describes it, “moving around eating everything up.” Or maybe not: “We don’t know how much of the fuel is actually burned in this blue whirl and how much is just evaporated. We need to do measurements.”
Listen to a podcast based on an interview with the researcher:
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.