Like Holding a Piece of Sky
By Mark Miodownik
Aerogels are the lightest solids in existence. Despite that trait, their complex internal structure makes them strong and exceptional insulators.
Aerogels are the lightest solids in existence. Despite that trait, their complex internal structure makes them strong and exceptional insulators.

DOI: 10.1511/2015.112.60
One day in 1998 I walked into the lab just as one of the technicians was taking a piece of material out of the microscope. "I'm not sure if you’re allowed to see this,” he said, “so we’d better be on the safe side, otherwise I’m going to have to fill in a load of paperwork.” He quickly covered up the material.
I was working for the US government at the time, in a nuclear weapons laboratory in the desert of New Mexico. Being a British citizen I had only the basic security clearance, and so there were areas in the laboratory complex I couldn’t go. Most areas, in fact. But this was our lab, so the behavior of the technician was definitely odd. I knew better than to ask him more. This was the late 1990s, a time when Chinese espionage in US national laboratories was a very sensitive issue.
The material was extraordinary, and although I only saw a small fragment of it for a mere second, I found it impossible to forget. Day after day, the thought of the mystery material would pop into my head and I would wonder what on Earth it could be. The fact that I couldn’t talk to anyone about it made it all the more difficult to forget.
I remembered it as being transparent, yet strangely opalescent—like a hologram of a jewel: a ghost material. I had definitely seen nothing like it before. Had it, I wildly speculated, been salvaged from some alien spacecraft?

I didn’t see it again until a few years later. I was back in the United Kingdom, having taken a job as the head of the Material Research group at King’s College London. One afternoon they announced on the TV news that on January 2, 2004, the NASA mission to capture stardust had successfully engaged with the comet Wild 2. The news program then showed a picture of my material. Well, obviously not my material, but the material I desperately wanted to be mine. “So it was alien!” I said triumphantly to my empty flat, as I scrambled on to my computer to find out more. “They are harvesting it from space,” I thought. Wrongly.
The material turned out to be a substance known as aerogel. I had got the wrong end of the stick from the news report: It was the aerogel that was being used to collect the stardust. I didn’t really stop to think about this but plowed on, collecting information about aerogels and their history. Aerogels were not of alien origin, I found out, but they nevertheless had a very strange back-story: They were invented in the 1930s by a man called Samuel Kistler, an American farmer turned chemist, who conjured them into existence solely to satisfy his curiosity about jelly. Jelly?
What was jelly? he asked. He knew that it wasn’t a liquid, but it wasn’t really a solid either. It was, he decided, a liquid trapped in a solid prison, but one in which the prison bars were like an invisibly thin mesh. In the case of edible jelly, the mesh is made from long molecules of gelatin, which is derived from the protein, collagen, that makes up most connective tissues, such as tendons, skin, and cartilage. When added to water, these gelatin molecules unravel and connect with one another to form a mesh that traps the liquid within it and prevents it from flowing. Jelly is basically like a water balloon, but instead of being an outer skin that holds the water within, it inhabits the water from the inside.
The water is held inside the mesh by a force known as surface tension—the same force that makes water feel wet and form drops, and causes it to stick to things. The surface tension forces inside the mesh are strong enough for the water to be unable to escape the jelly but weak enough for it to slosh around, which is why jelly wobbles. It’s also why jelly feels so amazing when you eat it: It’s almost 100 percent water, and with a melting point of 35 degrees Celsius the internal gelatin network promptly melts, freeing the water to burst in your mouth.
The simple explanation—a liquid trapped by a solid internal mesh—was not enough for Samuel Kistler. He wanted to know whether the invisible gelatin mesh within a jelly was all of a piece. In other words, was it a coherent, independent internal skeleton, such that if you could find a way to remove all of the liquid from it, the mesh could stand on its own?
To answer the question he conducted a series of experiments, the results of which he published in a letter to the scientific journal Nature in 1931 (3211[127]:741). The letter is entitled “Coherent Expanded Aerogels and Jellies,” and here is how he introduced the report:
The continuity of the liquid permeating jellies is demonstrated by diffusion, syneresis, and ultra- filtration, and the fact that the liquid may be replaced by other liquids of very diverse character indicates clearly that the gel structure may be independent of the liquid in which it is bathed.
Kistler died in 1975 having never seen his most wonderful material find a place in the world.
What Kistler is saying in this opening paragraph is that various experiments have shown that the liquid in a jelly is connected throughout, rather than being compartmentalized, and can be replaced by other liquids. This demonstrates, in his opinion, that the solid internal skeleton may indeed be independent of the liquid in the jelly. And in using the word “gel,” as a more general word for jelly, he is saying that this is true of a whole range of jellylike materials that span the gap between being truly solid and truly liquid, from hair gel, to solid chicken stock, to setting cement (where the internal mesh is formed by calcium silicate fibrils).
He goes on to point out that no one had yet managed to separate the liquid of a jelly from its internal skeleton:
Hitherto the attempt to remove the liquid by evaporation has resulted in shrinkage so great that the effect upon the structure may be profound.
In other words, those in the past who have tried to remove the liquid by evaporation have found that the internal skeleton simply collapses. He then goes on to say triumphantly that he and his collaborators have found a way to do it:
Mr. Charles Learned and I, with the kindly assistance and advice of Prof. J. W. McBain, undertook to test the hypothesis that the liquid in a jelly can be replaced by a gas with little or no shrinkage. Our efforts have met with complete success.
Their cunning idea was to replace the liquid with a gas while it was still inside the jelly, and so use the pressure of the gas to keep the skeleton from collapsing. First, though, they found a way to replace the water in the jelly with a liquid solvent (they used alcohol), which would be easier to manipulate. The danger of using a liquid solvent was that it too would evaporate, but they found a way to stop it:
Mere evaporation would inevitably cause shrinkage. However, the jelly is placed in a closed autoclave with an excess of liquid and the temperature is raised above the critical temperature of the liquid, while the pressure is maintained at all times at or above the vapor pressure, so that no evaporation of liquid can occur and consequently no contraction of the gel can be brought about by capillary forces at its surface.
An autoclave is simply a pressure tank that can be heated. By increasing the pressure in the autoclave, the liquid inside the jelly is prevented from evaporating, even when the temperature is increased beyond its boiling point. The capillary forces he talks about, meanwhile, are caused by the surface tension of the liquid. Kistler speculates that when the liquid is gradually removed through evaporation, these same forces that hold the jelly together are responsible for tearing it apart. But when he raises the temperature of the whole jelly above the “critical temperature”—the point at which there is no difference between a gas and a liquid because both have the same density and structure—the whole liquid becomes a gas without going through the destructive process of evaporation. He says,
When the critical temperature is passed, the liquid has been converted directly into a permanent gas without discontinuity. The jelly has had no way of “knowing” that the liquid within its meshes has become a gas.
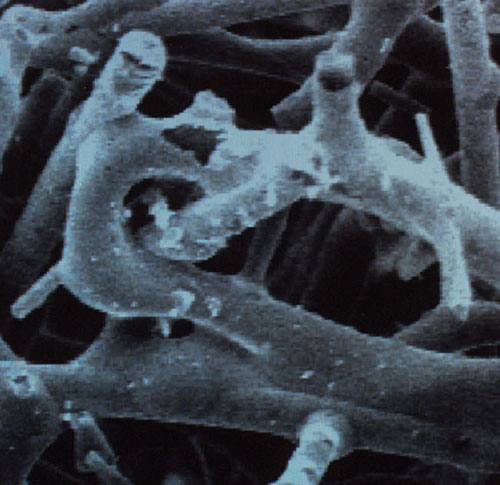
This is a stroke of genius: Under the pressure from the autoclave, the newly created gas cannot escape from the jelly and so the internal skeleton stays intact. “All that remains is to allow the gas to escape, and there is left behind a coherent aerogel of unchanged volume," he continues. Only now does he let the gas escape slowly, leaving the internal skeleton of the jelly completely intact and mechanically sound, thus proving his hypothesis. It must have been a very satisfying moment. But he didn’t stop there. These internal skeletons of jelly were incredibly light, fragile things, comprising mostly air. They were, in fact, foams. Perhaps he could make them stronger, he thought, by making a jelly not out of gelatin but out of something more rigid. So it was that he engineered a jelly in which the internal skeleton was made of the mineral silicon dioxide, the main constituent of glass. Using exactly the same process described above, he then created from this jelly a “silica aerogel,” the lightest solid in the world. This was the material I had seen for a split second all those years ago in a laboratory in the desert.
Not content with this achievement, Kistler went on to make other aerogels, and he lists them in the paper:
So far, we have prepared silica, alumina, nickel tartarate, stannic oxide, tungstic oxide, gelatine, agar, nitrocellulose, cellulose, and egg albumin aerogels and see no reason why this list may not be extended indefinitely.
Note that despite his triumph with silica aerogel he couldn’t resist making an aerogel from egg albumin—that’s egg white. So while the rest of the world were using egg whites to cook light fluffy omelets and bake cakes, Kistler did a different type of cooking using an autoclave to create egg aerogel: the lightest meringue in the world.
Silica aerogel looks extremely odd. Put it against a light background and it disappears almost entirely. In this sense, it is harder to see than normal glass, despite being less transparent. When light passes through glass, its path is distorted slightly—it is refracted—and the degree of distortion is known as glass’s refractive index. In the case of aerogel, because there is simply less of the stuff, light’s path is hardly distorted at all. For this same reason, there is no hint of reflection on its surfaces, and because of its ultra-low density it appears to have no distinct edges, to not be fully solid at all. Which of course it isn’t. The internal skeleton of a jelly has a structure not unlike that of bubble bath foam, with one main difference, which is that all of the holes link up. Silica aerogel is so full of holes that it is typically 99.8 percent air and has a density only three times greater than air, which means that it has practically no weight at all.
At the same time, when placed against a dark background silica aerogel is undoubtedly blue. And yet, because it is made from clear glass, it ought to have no color at all. For many years, scientists wondered why this might be. The answer, when it came, was rather satisfyingly odd.
When light from the Sun enters the Earth’s atmosphere, it hits all sorts of molecules (mostly nitrogen and oxygen) on its way down and bounces off them like a pinball. This is called scattering, which means that on a clear day, if you look at any part of the sky, the light you see has been bouncing around the atmosphere before coming into your eye. If all light was scattered equally, the sky would look white. But it doesn’t. The reason is that the shorter wavelengths of light are more likely to be scattered than the longer ones, which means that blues get bounced around the sky more than reds and yellows. So instead of seeing a white sky when we look up, we see a blue one.
This Rayleigh scattering, as it is called, is very slight indeed, so you need an enormous volume of gas molecules to see it: The sky works but a room full of air doesn’t. Put another way, any one bit of the sky doesn’t look blue but the whole atmosphere does. But if a small amount of air happens to be encapsulated in a transparent material that happens to contain billions and billions of tiny internal surfaces, then there will be sufficient Raleigh scattering off these surfaces to change the color of any light that passes through it. Silica aerogel has exactly this structure, and this is where its blue hue comes from. When you hold a piece of aerogel in your hand, it is, in a very real way, like holding a piece of sky.
Aerogel foams have other interesting properties, the most remarkable of which is their thermal insulation—their ability to act as a barrier against heat. They are so good at this that you can put the flame of a Bunsen burner on one side of a piece of aerogel and a flower on the other and still have a flower to sniff a few minutes later.
Because it is a foam, aerogel has within it the equivalent of a billion billion layers of glass and air between one side of the material and the other. This is what makes it such a superb thermal insulator. Having discovered this and other remarkable properties, Kistler reported them in the final sentence of his paper as follows:
Apart from the scientific significance of these observations, the new physical properties developed in the materials are of unusual interest.
Unusual interest, indeed. He had discovered the best insulator in the world.
The scientific community applauded briefly, but then promptly forgot all about aerogels. It was the 1930s and they had other fish to fry; it was hard to know what would shape the future and what would be forgotten. The world of materials was exploding and materials scientists would soon deliver nylon, aluminum alloys, silicon chips, fiberglass, and many other revolutionary materials. Somehow in all the excitement aerogels got lost and everyone forgot about them.
Everyone except one man, Kistler himself. He decided that the beauty and thermal insulation properties of these jelly skeletons were so extraordinary that they should and must have a future. Although silica aerogel is as fragile and brittle as glass, for its weight (which is minuscule) it has good strength—certainly enough to make it industrially useful. So he patented it and sold the license to manufacture it to a chemical company called Monsanto Corporation. By 1948 it was making a product called Santogel, which was a powdered form of silica aerogel.
Santogel seemed to have a bright future as the best thermal insulator in the world, but alas the time was not right for it. Energy was getting cheaper and cheaper, not more expensive, and there was no awareness of the problem of global warming. An expensive thermal insulator such as aerogel just didn’t make economic sense.
Having failed to find a market in thermal insulators, Monsanto rather bizarrely found applications for it in various inks and paints, its role being to flatten them optically by scattering light, creating a matte finish. Aerogel finally ended up being used ignominiously, as a thickening agent in screw-worm salves for sheep and in the jelly used to create napalm for bombs. In the 1960s and 1970s, cheaper alternatives usurped aerogel even from this rather limited repertoire of applications, and finally Monsanto gave up making it altogether. Kistler died in 1975 having never seen his most wonderful material find a place in the world.

The revival of aerogels came not as a result of any commercial application but because their unique properties attracted the attention of some particle physicists at CERN studying something called Cherenkov radiation. This is the radiation given off by a subatomic particle when it travels through a material faster than light can travel through it. Detecting and analyzing this radiation gives clues to the nature of the particle and so provides a very exotic means of identifying which of the many invisible particles the scientists are dealing with. Aerogel is extremely useful for this purpose—providing a material through which the particle can travel—as it is, effectively, a solid version of a gas, and it continues to be used for this today, helping physicists unravel the mysteries of the subatomic world. Once aerogels found their way into physicists’ labs, with their sophisticated equipment, esoteric aims, and big budgets, the material’s reputation started to grow again.
At that time in the early 1980s, aerogels were so expensive to make that they could only live in labs where money was no object. CERN was one such lab, but soon NASA followed. The first applications of silica aerogels in space exploration were to insulate equipment from extreme temperatures. Aerogels are particularly suitable for this application because not only are they the best insulators in the world, but they are also extremely light, and when you’re launching spacecraft out of the gravitational pull of the Earth, reducing weight matters rather a lot. Aerogel was used first in 1997 on the Mars Pathfinder mission and has been used as an insulator on spacecraft ever since. But once the scientists at NASA found that aerogel could cope with space travel, they realized that the material had another possible use.
If you look up into the sky on a clear night you might see a shooting star, which appears as a bright trail of light crossing the sky. For a long time it has been known that these are meteors that enter the Earth’s atmosphere at high speeds and burn brightly as they heat up. It is thought that most of these are space dust, which is leftover material from the creation of the Solar System 4.5 billion years ago, along with comets and other asteroids. Determining exactly what materials these heavenly bodies are made from has been of interest for many years, because this information could help us understand how the Solar System was formed and may also account for the chemical composition of the Earth.
Analyzing the composition of meteorites has given us some tantalizing clues, but the problem with these specimens is that they have all been heated to extremely high temperatures by their passage through the atmosphere. Wouldn’t it be nice, the people at NASA thought, if they could capture some of these objects out in space and bring them back to Earth in a pristine state?
The first problem with this idea is that objects in space tend to be traveling rather fast. Space dust is often going at speeds of 50 kilometers per second, or 18,000 kilometers per hour, a lot faster than a bullet. Catching an object like that is not easy. As with stopping a bullet with, say, your body, either the force of the bullet exceeds the rupture pressure of your skin, meaning it goes through you, or you employ a bulletproof vest made of a high-rupture-strength material, such as Kevlar, which results in a compressed and deformed bullet. Either way, it’s a risky business. However, in principle, it is quite possible—just as when catching a cricket ball or baseball with “soft” hands, the trick is to spread and dissipate the ball’s energy rather than bracing yourself for a single, high-pressure impact.
What NASA needed, then, was a way to slow the dust down from 18,000 kilometers per hour to zero without damaging the dust or the spacecraft—ideally a material with a very low density, so that the dust particles would be slowed gently without being damaged; ideally one that could do so within the space of a few millimeters; and ideally one that would be transparent, so that scientists could find the tiny specks of dust once they were buried in it.
That such a material existed was a minor miracle. That NASA had already used it in space flights was extraordinary. It was, of course, silica aerogel. The mechanism by which aerogel pulls off this feat is the same as the one used to protect stunt actors in movies when they fall off tall buildings: A mountain of cardboard boxes, each box absorbing some of the energy of the impact as it collapses beneath the actor’s weight, and the more boxes, the better. In the same way, each foam wall within aerogel absorbs a tiny amount of energy when it is struck by the dust particle, but because there are billions of them per cubic centimeter, there are enough of them to bring it to a halt relatively unharmed.
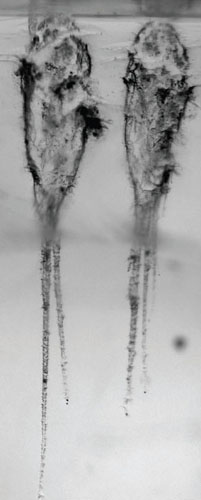
NASA built an entire space mission around the ability of aerogel to gently collect stardust. On February 7, 1999, the Stardust spacecraft was launched, containing all of the equipment necessary to take a trip through the Solar System, while also being programmed to fly past a comet called Wild 2. The idea was that it would collect interstellar dust from deep space as well as the dust being ejected from a comet, allowing NASA to study the material composition of both. In order to do this, they developed a tool that resembled a giant tennis racket, but instead of holes between the strings there was aerogel.
During the summer and autumn of 2002, while millions of kilometers from any planet, the Stardust spacecraft opened a hatch and poked out its giant tennis racket fitted with aerogel. It had no opponent in this game of interstellar tennis and the balls it was looking for were microscopically small: the remains of other stars long gone, the leftover ingredients of our own Solar System still flying around. The Stardust spacecraft couldn’t hang around in deep space too long because it had an appointment to keep with the comet Wild 2, now hurtling from the outer reaches of the Solar System and approaching the center, which it does every 6.5 years.
Having withdrawn its aerogel tennis racket, the spacecraft sped off for its meeting. It took just over a year to get to the right position, but on January 2, 2004, the spacecraft found itself on a collision course with the comet, which was 5 kilometers in diameter and speeding off around the Sun. Once it had maneuvered itself into the slipstream of the comet, 237 kilometers behind it, the spacecraft opened its hatch and once again poked out its aerogel tennis racket, this time using the B-side, and started to collect, for the first time in human history, virgin comet dust.
After collecting the comet dust, the Stardust spacecraft returned to Earth, arriving back two years later. As it approached the Earth it veered away, jettisoning a small capsule, which fell under Earth’s gravity, entering the atmosphere at a speed of 12.9 kilometers per second, the fastest re-entry speed ever recorded, and so becoming for a while a shooting star itself. After 15 seconds of free fall, and having reached red-hot temperatures, the capsule deployed a drogue parachute to slow down the rate of descent. A few minutes later, at a height of 10,000 feet above the Utah desert, the capsule jettisoned the drogue chute and deployed the main parachute. At this point the recovery crews on the ground had a good idea of where the capsule was going to land and headed out into the desert to welcome it back from its 7-year, 4-billion- kilometer round trip. The capsule hit the sand of the Utah desert at 10:12 GMT on Sunday, January 15, 2006.
Until they opened the capsule and started examining the aerogel samples, scientists had no idea whether they held any answers to anything. Perhaps the space dust would have passed straight through the aerogel. Or perhaps the violence and deceleration of re-entry would have disintegrated the aerogel into meaningless powder. Or perhaps there would be no dust at all.
They need not have worried. Once they got the capsule back to the NASA laboratories and opened it up, they found that the aerogel was fully intact and almost completely perfect. There were minuscule puncture marks in the surface and it was these that were subsequently shown to be the entry points for the space dust. Aerogel had done the job that no other material could do: It had brought back pristine samples of dust from a comet formed before the Earth even existed.
Since the return of the aerogel capsule, it has taken NASA’s scientists many years to find the tiny pieces of dust embedded within the aerogel, and the work continues to this day. The dust they are looking for is invisible to the naked eye, and so it must be found by microscopic examination of the samples, which has taken years. The project is so massive that NASA has enlisted the public to help with the search. The scheme Stardust at Home trains volunteers to use their home computers to look through thousands of microscopic images of the aerogel samples and try to spot the signs that a piece of space dust is present.
The work so far has thrown up a number of interesting results, the most surprising of which is that most of the dust from the comet Wild 2 shows the presence of aluminum-rich melt droplets. It’s very hard to understand how these compounds could have formed in a comet that had only ever experienced the icy conditions of space, because they require temperatures of more than 1,200 degrees to do so. Because comets are thought to be frozen rocks that date back to the birth of the Solar System, this has come as a bit of a surprise, to say the least. The results seem to indicate that the standard model of comet formation is wrong, or there is a lot more we don’t understand about how our Solar System formed.
Now that the Stardust mission is over, will this be the fate of aerogel too, to end in obscurity? It is all too possible. Although aerogels are the best insulators we have, they are very expensive and it is not clear that even now we care about energy conservation enough to value aerogels economically. There are several companies selling aerogel for such thermal insulation applications, but at the moment the main ones are for extreme environments such as drilling operations.
There is no weight to it that you can perceive, and its edges fade away so imperceptibly that it is impossible to see where the material stops and the air begins.
Research on developing new aerogels has been taking place at an increasingly rapid pace. There are now a number of aerogel technologies that result in a material that is not rigid and brittle, as silica aerogels are, but flexible and bendy. These so-called x-aerogels are made flexible by a neat piece of chemistry that detaches the rigid foam walls of an aerogel from one another and inserts between them polymer molecules that act like hinges within the material. These x-aerogels can be made into flexible materials such as textiles and could be used to make the warmest but lightest blankets in the world, potentially replacing duvets, sleeping bags, and the like. Because they are so light, they would also be perfect for outdoor clothes and boots designed for extreme environments. They could even replace the foam soles in sports shoes that make that type of footwear so springy. Recently, a family of carbon aerogels has been created that conduct electricity, as well as super-absorbent aerogels that can suck up toxic waste and gases.
Aerogels may yet be part of our everyday lives, the answer perhaps to living in a more extreme and volatile climate. But although as a materials scientist it’s good to know that we are likely to have the right materials to offer the world in the event that global warming is not averted, this is not the kind of future I want for my children. In a world where we have industrialized so many materials (including those we used to hold sacred, such as gold and diamond), I like to think there may again be a place for a material valued solely for its beauty and significance.
Most people will never hold a piece of aerogel in their hand, but those who do never forget it. It is a unique experience. There is no weight to it that you can perceive, and its edges fade away so imperceptibly that it is impossible to see where the material stops and the air begins. Add to this its ghostly blue color and it really is like holding a piece of sky. Aerogels have the ability to compel you to search your brain for some excuse to be involved with them. Like an enigmatic party guest, you just want to be near them, even if you can’t think of anything to say. These materials deserve a different future, not of oblivion or embedment in a particle accelerator, but to be valued for themselves.
Aerogels were created out of pure curiosity, ingenuity, and wonder. In a world where we say we value such creativity, and give out medals to reward its success, it’s odd that we still use gold, silver, and bronze to do so. If ever there was a material that represented humankind’s ability to look up to the sky and wonder who we are, if ever there was a material that represented our ability to turn a rocky planet into a bountiful and marvelous place, if ever there was a material that represented our ability to explore the vastness of the Solar System while at the same time speaking of the fragility of human existence, if ever there was a blue-sky material—it is aerogel.
(Excerpted from STUFF MATTERS by Mark Miodownik. Reprinted by permission of Viking Penguin and Houghton Mifflin Harcourt Publishing Company. Copyright © 2013/2014 by Mark Miodownik. All rights reserved. This selection may not be reproduced, stored in a retrieval system, or transmitted in any form by any means without prior written permission of the publisher.)
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.