Wiggling Through the World
By Daniel I. Goldman, David L. Hu
The mechanics of slithering locomotion depend on the surroundings
The mechanics of slithering locomotion depend on the surroundings

DOI: 10.1511/2010.85.314
Movement is critical for the survival of animals. Eels swim, hawks soar, moles tunnel and squirrels leap to perform vital tasks such as foraging, mating and escaping predation.
These feats of locomotion involve the coordination of complex biophysical processes that span scales from the tiny (ion channels in nerve fibers that depolarize to send and receive information) to the intermediate (muscles and tendons coupling to skeletal elements to generate motion of body parts) to the large (interaction of body parts such as feet and hands with their surroundings to effectively generate traction).
A goal of locomotion science is to uncover general principles of movement through the development of models across sizes. This challenge requires the collaboration of biologists, physicists, mathematicians and engineers. Such animal- locomotion studies are also inspiring the design of vehicles with mobility equal to or greater than that of animals.
Many animals move effectively without the use of limbs. Some legged lizards, for example, are known to forsake their limbs entirely and wiggle their bodies to move through dense grasses or sandy environments. For other creatures, such as the thousands of species of snakes, slugs and worms (and a few lizards), legs were so superfluous that they have been completely limbless for millions of years. Their long, flexible bodies enable them to enter tight crevices and to traverse long distances through complex and often tortuous substrates such as the tops of trees, underneath the soil or inside the digestive tracts of other organisms.
Our recent laboratory work and models of terrestrial limbless locomotion have elucidated the mechanisms that make undulatory locomotion effective in two distinct environments: above ground where snakes slither (Hu) and within flowing substrates such as sand where sandfish lizards “swim” (Goldman). We hope to give a glimpse of how such animals can move at speeds of several body-lengths per second by describing the movement of snakes and sandfish in turn, and drawing attention to the specific adaptations these animals use to enhance their performance in particular habitats. Although the motion of these snakes and sandfish may appear similar, these animals propel themselves using substrate interactions that are distinct to their respective environments, as we have determined with mathematical and physical modeling.
The common features of our approaches to mathematical modeling are derived from applying what’s called the resistive-force technique, developed to model the locomotion of small organisms in fluids. Consider a small rod-shaped “slice” of an undulating reptile. Muscular forces acting on the segment, combined with the inertia of the body, generate equal and opposite reaction forces from the animal’s environment. If the animal moves its body in particular ways, the sum of all the reaction forces on the animal’s body can propel the animal’s center of mass forward. To simplify our model, we will assume that our animals undulate in a plane. Thus we can decompose the force acting on the segment into two pieces, one tangential and the other perpendicular to the segment’s surface. These are called the axial and normal forces, respectively (see Figure 2). For an undulating animal to move, the summation of the forward components of the normal forces on the animal must exceed the backward components of the axial forces. To determine if that is the case, we first must calculate the reaction forces from the animal’s environment. Although mathematically this approach seems straightforward, it requires input of models of the environment, and this is where the new modeling challenge begins.
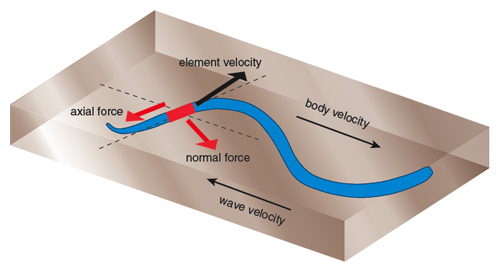
Illustration by Tom Dunne
In fluid environments, such as those typically encountered by swimming spermatozoa, nematodes or sea snakes, we can use what are called the Navier-Stokes equations to account for force produced by flows. These equations, named after physicists Claude-Louis Navier and George Gabriel Stokes, apply Newton’s second law to fluid motion, and account for pressure and viscosity, in order to describe fluid movement. However, these partial differential equations are often impossible to solve analytically, so there are many approximate models (such as Stokes’ Law for viscously dominated disturbances) that can be used to rapidly calculate forces experienced by swimmers and fliers.
In contrast, environmental models for terrestrial locomotion can be more complex. Although dry friction can describe interactions with some surfaces, we lack validated equations for many materials such as mud and sand. For surfaces that can be approximated by Coulomb (or dry) friction, resistance force is independent of speed, proportional to the applied normal force and opposite to the direction of motion. The response of a dry, granular material such as sand can be like a solid or a fluid, depending on applied stresses and the compaction of the material. Moreover, the compaction can change after a disturbance and thus drag resistance in granular media depends on the history of how it was perturbed.
Despite these differences, slithering locomotion appears to work well in water, on flat land and, as shown by one of our recent discoveries, even through granular material. Undulatory locomotion in dry environments, both above and below ground, has an important feature that makes the mathematics more tractable: The inertia of both the organism and the surroundings are negligible compared to frictional forces—thus to stop moving forward, the animal simply stops slithering. This is unlike a large snake swimming in water—-if it stops undulating, it coasts for a distance related to its initial speed.
Snakes, and some snakelike lizards without legs, are a highly successful class of terrestrial limbless creatures. They have evolved to span three orders of magnitude in length, from the centimeter-scale threadsnakes to 10-meter-long anacondas. All possess the same basic body design: a flexible tube of flesh covered in hardened scales. This form provides them with tremendous versatility: They can slither vertically up tree trunks, transition from slithering on land to swimming in water without changing gait, travel on land using a similar amount of energy to a legged organism of the same weight, and some, such as the 2-meter-long black mamba, sprint nearly as fast as a human can run (5 meters per second).
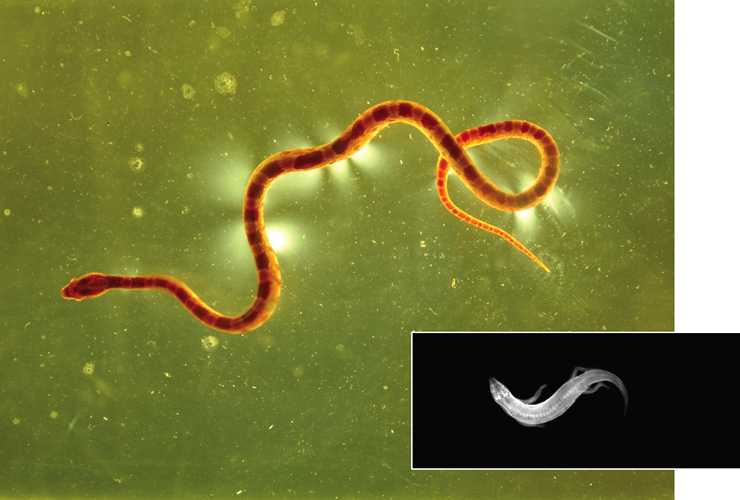
Working in 2009 with Michael Shelley of New York University, one of us (Hu) focused on the locomotion of juvenile milk and corn snakes because of their ability to slither in terrestrial habitats such as prairies and rocky slopes. Like all snakes, they are capable of several gaits, or sequences of placements of their limbless body on the ground. We investigated the most common of their limbless gaits, slithering. This gait is also known to biologists as lateral undulation, and its utility to locomotion in snakes has been previously described on the basis of so-called push points: Snakes slither by driving their flanks laterally against neighboring rocks and branches found along the ground. Thus, early experiments involved snakes slithering through a pegboard. A snake can generate forward motion on the boards because its combined muscular forces on the surrounding pegs exceeds the sliding friction force on its belly. However, snakes can also slither easily on relatively featureless terrain, such as sand or bare rock, which do not provide obvious push points. We sought to understand this physical regime in our experiments, in part because of the relative ease with which frictional interactions could be incorporated into our mathematical modeling.
Daniel I. Goldman and David L. Hu
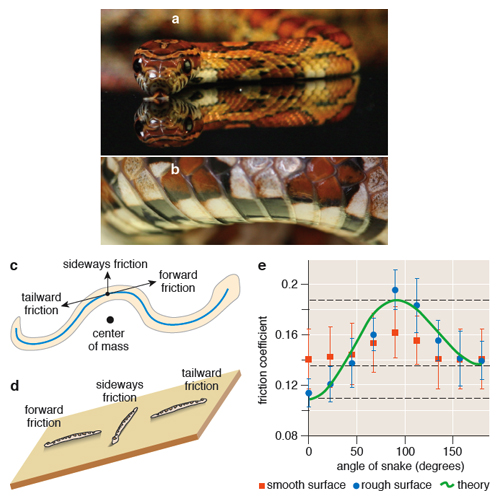
Friction is a familiar force that resists the sliding of one object atop another, such as the soles of our shoes on the ground. The force arises from the fact that no surface is completely flat: Under a microscope, all surfaces are covered with tiny peaks and valleys, called asperities, that snag and deform when two surfaces are pressed together and slid. This resistance to sliding is described by a friction coefficient, the ratio between the resultant friction force between two surfaces and the compressive force applied. For example, for a snake to slither (or equivalently, in order to drag a sleeping snake along the ground), the snake must apply a force greater than the product of the friction coefficient and the weight of the snake.
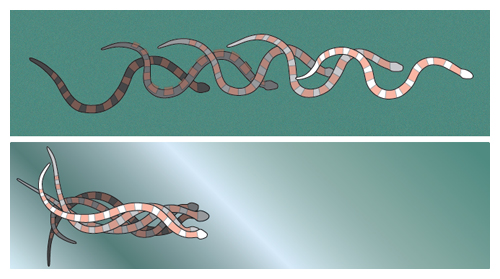
Although snakes’ backs are covered with diamond-shaped scales, their belly scales are arranged like the overlapping shingles on the roof of a house, which snag on the ground when the snake is slid sideways or tailward. This orientation gives snakes a handy frictional property: On certain substrates, their belly scales have a preferred direction of sliding. By putting snakes to sleep for a few minutes (with low doses of anesthetic gas) and straightening them out in various orientations (head down, head up and sideways) on inclined planes, we measured the snake’s friction coefficient and its dependence on the orientation of the snake. Friction measurements were performed on cloth fabric whose characteristic length scale of roughness (0.2 millimeters) was comparable to the thickness of the snake’s belly scales (0.1 millimeters), enabling the scales to snag in the cloth. Measurements of milk snakes on this cloth indicate the friction coefficient is lowest if the snake slides forward (0.10), intermediate if sliding tailward (0.14) and highest towards its flanks (0.20). This response is called frictional anisotropy (a physical property whose value depends upon the direction in which it is measured), without which the snake would be unable to move forward on flat ground.
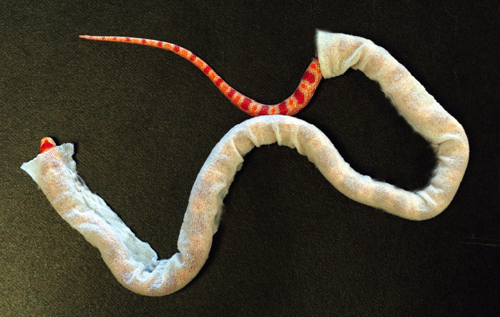
The necessity of snake scales to locomotion can be shown by dressing snakes in an “isotropic jacket;” in our work, this is a sleeve of fabric snug enough to cling to the snake without impeding its breathing. Friction forces still resist the jacketed snake’s sliding on the ground, generating forces in both the normal and axial directions of the belly. However, the friction-force magnitude is now equal in every direction. When the snake generates a traveling wave, the forces on the snake’s belly sum to zero. Physically, this means that the snake slithers in place, as if it were on slippery ice. The same effect can be achieved by placing snakes on very smooth surfaces such as plastic. On such substrates, snakes are unable to progress forward unless they lift their bodies while slithering or transition to another gait (such as sidewinding, or concertina mode, in which the snake folds itself like the pleats of an accordion).
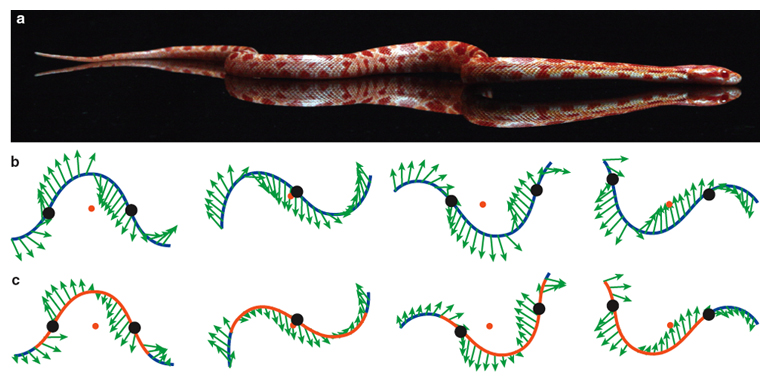
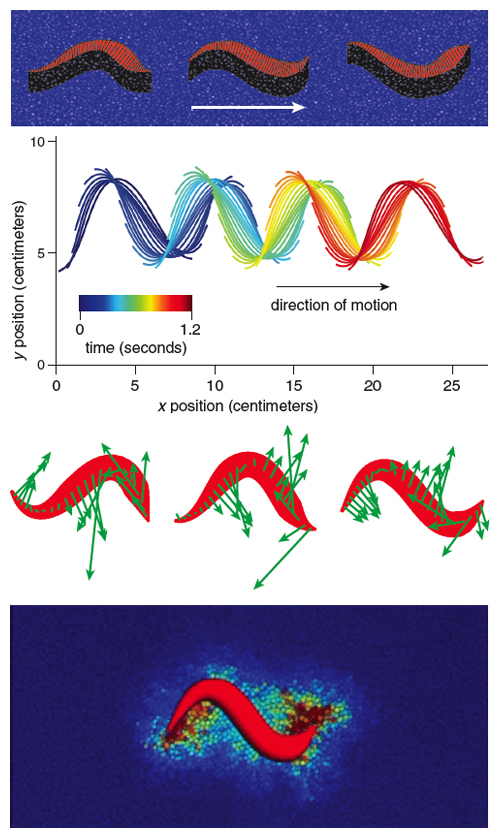
In our mathematical model, we used the snake’s scale properties to determine the steady speed of the snake’s center of mass. The inputs to the model include the belly friction coefficients and characteristics of the snake’s undulation kinematics (frequency, wavelength and amplitude). We assume that along the snake’s length, its weight is uniformly applied to the ground. Figure 6 shows our virtual snake juxtaposed by green arrows denoting the magnitude and direction of the friction forces acting on the snake’s belly. The components of the arrows pointing in the snake’s direction of motion are responsible for its propulsion; the remaining components indicate directions in which the snake’s energy is wasted. Upon adding all the frictional forces, we were surprised to find that the speed of our virtual snake was only half that of the snake we observed in the lab (8 centimeters per second, or 0.2 body lengths per second). Some part of snake behavior clearly was not being captured in our simplified mathematical model.
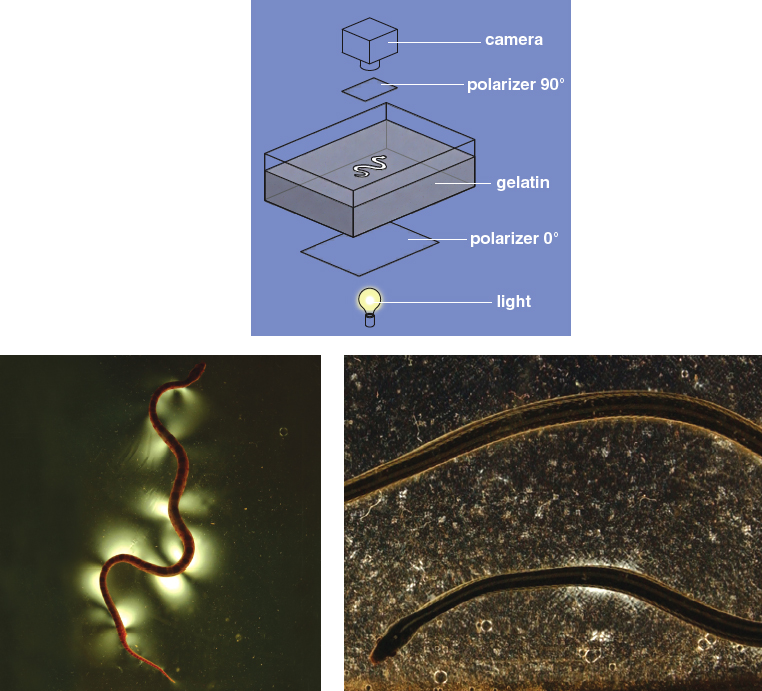
Previous investigators have observed snakes altering their weight distribution by lifting the peaks and troughs of their undulating bodies, concentrating their weight on the remaining points of contact with the ground. This body lifting is most clear in sidewinding, in which the snake travels laterally and leaves a trail in sand that resembles discrete “footsteps’’ rather than a continuous winding path. Our experiments with snakes on mirrored surfaces and photoelastic gelatin (which transmits light when compressed) indicate that snakes are also capable of lifting their bellies while they slither forward.
In our model, we showed theoretically that such dynamic load balancing leads to increases in speed of 35 percent and in efficiency of 50 percent. Why such a large advantage? Figure 6 shows the directions of propulsive forces (friction) everywhere along the snake. The peaks and troughs of the curves show propulsive-force arrows that point normal to the direction of snake motion, the direction in which energy is wasted. Because friction is proportional to the weight applied, the snake generates more thrust if it lifts its body in these regions and increases its weight elsewhere. Thus, slithering shares certain features with human walking. When we walk, we transfer weight from our hind foot to the leading foot by lifting the hind foot rather than dragging it. Similarly, a snake lifts the parts of its body that are doing the least useful work. This adjustment is a simple change to their weight distribution that snakes can perform. By working to understand weight distributions further, we may one day know how speedier snakes such as the black mamba can move so quickly.
Many desert organisms, including snakes, moles, lizards and scorpions, disappear into sand to avoid predators and heat as well as to catch prey. The sandfish, which one of us (Goldman) studies, is a 10-centimeter-long desert lizard with fringed toes on its four limbs, a shovel-shaped snout and a flattened belly and flanks—these features are hypothesized to aid it in burying itself and sand-swimming. Like snakes, its scales are smooth and abrasion resistant. Unlike snakes, the belly scales on the sandfish do not overlap. Although there have been many hypotheses about how such organisms move within a medium, until our work there had been almost no detailed studies of kinematics, in part because opaque sand makes subsurface visualization challenging.

Daniel I. Goldman and David L. Hu
The sandfish uses its limbs to move rapidly on the surface of the sand but when startled, it points its snout down and quickly disappears (within half a second) beneath the sand. Once fully submerged, the sandfish is quite challenging to locate. The physics that governs the propulsive forces in the granular world into which the sandfish descends are quite different from the frictional forces that are important for slithering on solid surfaces, mainly because granular materials such as sand can yield (flow) and solidify in response to perturbation.
Desert sand is typically dry and composed of roughly spherical particles from 0.1 to 0.3 millimeters in diameter that only interact on contact through energy-dissipating forces such as viscoelasticity, plastic deformation and friction. Depending on the applied stress, granular media can display a range of physical behaviors with features characteristic of gases, fluids and solids. For example, a pile of grains on a flat board behaves like a yield-stress fluid: It acts like a solid if the pile is not tilted too much, but at sufficiently high angles of inclination it undergoes a transition to a fluid that flows downhill. There is not yet a fundamental comprehension of the mechanics of these materials at the level known for fluids such as water and air.
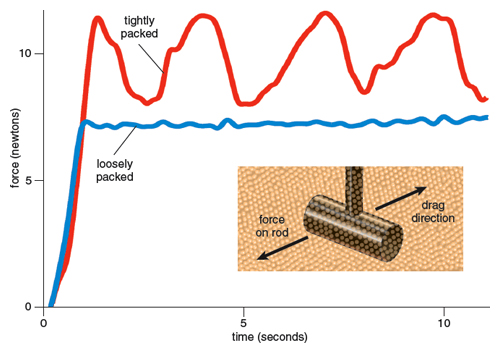
The behavior of granular media is sensitive to their conditions and preparation: One of us (Goldman) has recently investigated how a parameter called the packing fraction—the ratio of the material volume of grains to the occupied volume—controls the material’s response to sustained perturbation (such as the movement of an object through the sand). Although the range of naturally occurring disordered packings occupied by approximately spherical dry grains is small (58 percent at the loosest and 63 percent at the most tightly packed), these different states behave quite differently: A loosely packed collection of grains behaves in a more fluidlike manner, flowing smoothly in response to disturbance, whereas in a tightly packed collection of grains the drag force nearly doubles and the grains flow in a halting, abrupt manner when an object is dragged through them. This is a consequence of the ability of loose packings to flow by particles pushing into free volume, whereas tight packings flow as groups of particles create new volume by expansion (called dilation). Unlike in fluids such as water, in which force on a moving object increases with rising velocities, in granular media for low enough velocities, forces on objects are approximately independent of speed, because velocity-independent frictional interactions dominate the particle interactions in this regime.
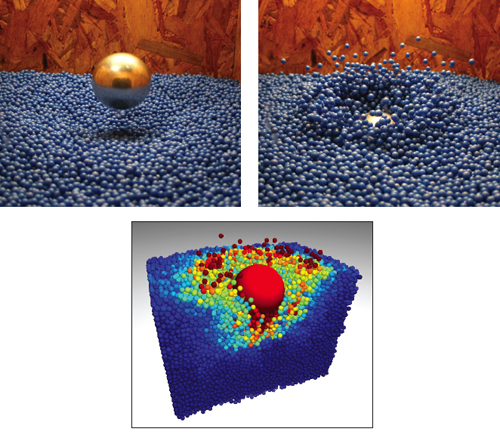
Modeling such behavior is also a challenge. Although there has been much progress in describing the gaslike state of shaken granular media, drag laws are not available for flows in which fluid and solid states coexist. A successful approach to modeling these materials is to instead apply a more “brute force” approach—let a computer follow the motion and interaction of millions of individual grains subject to collision rules and gravity. This procedure is called molecular dynamics. Once validated, such models can give insight into particle-level flows, functioning as a virtual microscope into the medium.
It is fascinating to contemplate how a sandfish moves within sand. Does the animal use its limbs to paddle or does it undulate like an eel, or both? Once its head breaks up the material, does it remain a fluid or does it solidify fast enough that subsequent portions of the body have to refluidize it? Do changes in material compaction (loosely to tightly packed sand) change the behavior and movement pattern of the sandfish? To investigate questions such as these, one of our (Goldman’s) doctoral students, Ryan Maladen, uses a combination of x-ray imaging techniques to visualize subsurface motion.
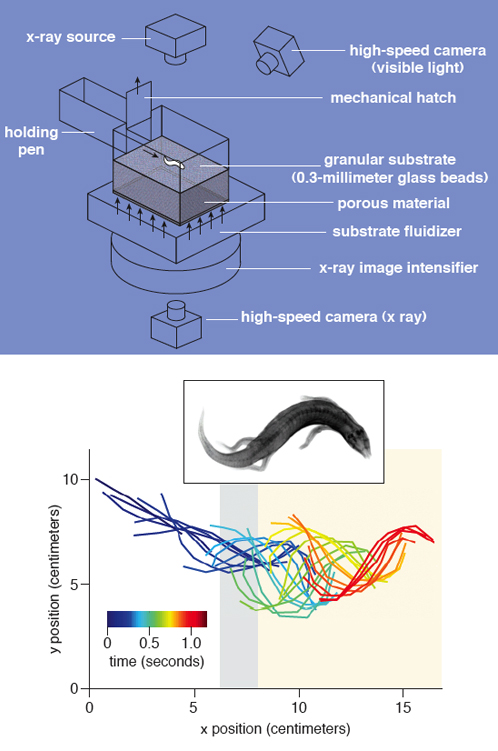
To control the properties of the sand encountered by the animal, we use a custom-made fluidized bed, a device in which a collection of grains placed into a container with a porous bottom is driven upward by a flow of air. Below a critical flow rate, the grains remain in a solid state, but above this threshold, the grains take on the properties of a fluid. Once the airflow is stopped, the grains settle into a loosely packed state; subsequent perturbations by either pulses of air or controlled vibrations to the bed can create repeatable states of different compaction.
During experiments, the sandfish is placed in a holding pen connected to the bed, then a gate on the pen is lifted. Once the animal realizes it has access to the sand, it immediately runs out of the pen toward the material, with its back straight and using its limbs for propulsion. High-speed video reveals that to bury itself the animal uses a combination of its limbs and its body to push itself into the sand. The burial time is rapid and does not depend significantly on the packing fraction of the sand.
Once below the surface of the sand, direct visualization with high-speed cameras become impossible. To image movement within the material, we rely on high-speed x-ray video. The sand (and the lizard) are placed between an x-ray source and a scintillating material (called an image intensifier), which converts the x-ray photons into electrons, which strike another surface that emits visible light, which is then captured by a conventional high-speed camera.
When we first observed the subsurface movement of the sandfish, we were amazed to see that it was moving forward at nearly two body lengths a second using a large-amplitude undulation of its body, and without using its limbs—traveling faster than milk snakes move on flat ground. The sandfish “swam” to a depth of about 4 centimeters and then stopped undulating, presumably feeling safe. The sandfish moved forward by propagating a traveling sinusoidal wave backward from head to tail at a particular frequency—the greater the frequency, the faster it went. In tightly packed media, the animal used frequencies up to four undulations per second.
There were several interesting features of the sandfish locomotion: We expected that, as we increased the packing fraction to generate more resistive media, the animal should slow down. Measurements we made on small rods in granular media showed that drag force in tightly packed sand is nearly double that in loosely packed sand. Surprisingly, we found that volume fraction had no effect on speed for a given wave frequency. Further, the animal’s movements were the same in both loosely and tightly packed media; it was impossible to determine simply by looking at the x-ray images if the animal was swimming in more or less resistive materials. Stranger yet, we found that on average, the sandfish swam faster in the tightly packed material than in the loosely packed material. It did this by increasing undulation frequency to a higher range (its maximum frequency was nearly doubled in tight versus loose packings).
In experiments and models, we use a number called the wave efficiency to characterize the locomotion of the sandfish—it is commonly used to characterize the locomotion of other swimmers (such as millimeter-long nematode worms) in deformable media. Wave efficiency is defined as the average swimming speed divided by the wave speed (which is a product of the frequency and the wavelength). If there is no movement of the material and no slip of the animal, the wave efficiency is one and the animal effectively moves in a tube. If the animal were in a vacuum with nothing to push against, the wave efficiency would be zero. In granular media, we found that the sandfish swam with a wave efficiency of 0.5, nearly twice that of nematodes in fluids (about 0.2) and greater than snakes on frictional surfaces (about 0.3). Remarkably, the wave efficiency of the sandfish did not depend on the compaction of the sand.
To explain some of these phenomena, a doctoral student in one of our (Goldman’s) groups, Yang Ding, first applied the resistive-force technique (RFT) described above for snakes. We hypothesized that this approach would be applicable in sand because the x-ray images indicated that the material near the animal was flowing. Also, dissipation in granular media is large and disturbances from different regions of the sand do not influence each other so that forces from separate elements (slices) of the body can be linearly combined.
Unlike the frictional interaction that describes snake locomotion, the appropriate force laws for granular media were unknown. Ding and Chen Li, another doctoral student in the group, developed empirical force laws by measuring the drag components on a stainless-steel cylinder (that approximated the skin friction of the sandfish) as it was pulled through sand at different angles relative to the displacement direction. The axial law resembled that of a true fluid, but the normal force law was “enhanced” relative to what we would have predicted from a true fluid or from pure Coulomb friction. However, as we expected, both forces were independent of the magnitude of drag speed.
We hypothesized that the enhancement of the normal force in granular media was responsible for the increased wave efficiency of the sandfish compared to animals such as nematodes that use viscous-fluid forces or snakes that use friction forces. Indeed, when the drag laws were inserted into the RFT and integrated using the animal’s measured movements, the model correctly predicted that speed increased linearly with frequency. Moreover, the predicted wave efficiency was in a range of 0.4 to 0.7, independent of volume fraction. This result bounded the measured sandfish wave efficiency, and its range was a consequence of not accurately knowing the drag on the sandfish’s shovel-shaped snout (we made predictions for minimal wave efficiency for a flat-headed model and maximal efficiency for a model without a head).
Thus, the RFT demonstrated that the animal could indeed “swim” within sand without use of limbs. The medium in which it was swimming demonstrates fluidlike properties, although the flow is better described as a frictional fluid, as opposed to a viscous fluid. The RFT model gave a plausible explanation for the independence of wave efficiency on packing fraction: As resistance force increased in close-packed material, so did the thrust forces that could be generated. The enhanced normal force within the granular media was responsible for the high wave efficiency of the sandfish, which was even greater than that of a snake moving on a flat surface (without lifting). That the sandfish wave efficiency is higher than that of snakes is striking given that the sandfish must burrow its entire body through sand.
The RFT made an interesting prediction for optimal undulatory locomotion within a granular medium: The sandfish can increase its speed by increasing its wave amplitude while maintaining a wave of approximately a fixed single period (the number of undulations along the body from head to tail). But since the animal has a finite length, a larger amplitude leads to less forward progress at each cycle—the head moves closer to the tail so that at the limit of a large amplitude the animal is basically moving perpendicular to the direction of motion. The RFT predicted a maximum speed at amplitudes of about 0.2 of the wavelength. Remarkably, we found that the sandfish data clustered at this peak, indicating that our animals were determined to flee as rapidly as possible through the sand.
Although the agreement between the biological data and the RFT model was encouraging and the prediction of optimal swimming was tantalizing, the RFT approach suffers from some drawbacks. For one, it is challenging to change parameters—for example, if we wish to perform the analysis in beads of different size or with altered surface features (such as friction), we must remeasure the empirical force laws, a time-consuming task. In addition, it was not clear that certain assumptions in the RFT were valid during sand-swimming (such as the assumption that different segments did not influence each other and the use of a steady-state, constant-velocity drag force for oscillating segments) so perhaps the agreement was only fortuitous.
Tom Dunne, Daniel I. Goldman and David L. Hu
To rigorously test our ideas about optimal sand-swimming and to improve our modeling efforts, we took a second approach using the molecular-dynamics techniques described previously. The model for the interaction of the particles incorporates contact elasticity, viscous force during contact to model collisional energy loss (called the coefficient of restitution) and tangential interaction (assumed to be Coulomb friction), and can be calibrated by comparing the simulation to measurements of forces in experiments. Once these three parameters are determined, we find that the molecular dynamics model has good predictive ability over a range of experimental conditions. For example, interaction laws of grains measured at one drag velocity or angle predict the forces under all other conditions. In these experiments and simulations we used 3-millimeter glass beads instead of the 0.3-millimeter beads used in the previous sandfish experiments. This switch made simulation possible, because we could reduce the number of particles in our box by a factor of 1,000—simulations ran in a matter of days on our desktop computers instead of years. Experimentally, the sandfish swam in the larger beads using the same wave efficiency as in the 0.3-millimeter particles.
Once particle properties were known, we created a virtual sandfish with movement patterns taken from our biological experiments and forces calculated by molecular-dynamics simulation. We found that the molecular-dynamics modeling was in good agreement with the wave efficiency predicted by the RFT calculations (as well as measured in the biological data). Unlike the empirical RFT models, molecular dynamics allows access to particle-level information—we could visualize the highly damped flow of particles around the sandfish and estimate accurately the force on different elements. Additionally confirming our RFT model, the simulated sandfish moved fastest when it used the kinematics predicted by the RFT and taken from observations of the animal. Further work with the molecular-dynamics model will allow us to carefully investigate the physics that creates the scaling of thrust and drag, which leads to the independence of wave efficiency on packing fraction.
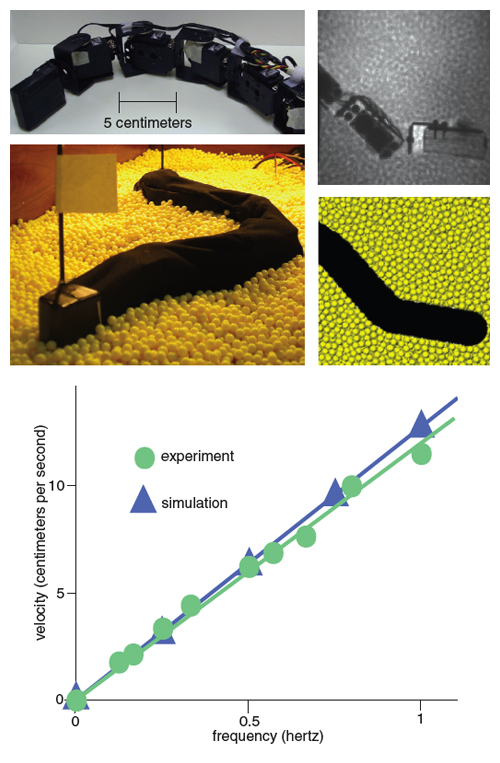
We wanted to give our models one more test, so we decided to move back into the physical world: We would use the molecular-dynamics simulation to design a physical model of the sandfish, a robot, that would swim within granular material and test the principles we had learned. For our tests, we chose segments made of servo- motors popular with hobbyists, so we were therefore constrained in size by commercially available actuators. We also decided to scale the size of the granular particles to 6 millimeters, so that we would not have to simulate billions of grains and small particles would not get into the motors. Ryan Maladen, in collaboration with mechanical engineer Paul Umbanhowar at Northwestern University, built a device that could undulate with the same wave pattern as that of the sandfish. The agreement between robot experiment and robot simulation was within five percent. We found, just as predicted by the models, that the robot swam fastest in the real world (and in simulation) using the optimal sandfish kinematics. The optimal wave efficiency of the robot sandfish was 0.3 in both experiment and simulation. We attributed this result to the finite number of segments of the robot—in the simulation, when we increased the number of segments so that the body was nearly smooth, the optimal wave efficiency approached 0.5.
We have studied two very different environments in which undulatory locomotion is effective. In above-ground snakes, anisotropic belly friction generates thrust to overcome drag, whereas underground, an animal’s sides exploit the frictional fluidlike properties of granular media to generate thrust. Although the drag laws differ between these regimes, we found that resistive-force modeling techniques, which originated in hydrodynamics, can be successfully applied to organisms on and within dry land. Our experiments and modeling considered mostly planar motion of the body, but our findings of the advantages of dynamic body-lifting in snakes suggests that motion in the third dimension may be used to increase performance. In the sandfish, we must begin to explore 3D effects as well: Our x rays reveal that the animal does not simply swim in a fixed horizontal plane, but actually dives into the material at a shallow angle.
Modeling the interaction between the organism and its environment enables us as physical scientists to work with biologists to uncover new behaviors and the relevant neuromechanics associated with effectively using long, slender bodies to move. The modeling approach does not constrain us to sandfish or snake morphology: Using our models of the animals, we can vary body shape and waveform to understand benefits and tradeoffs of different locomotor modes in diverse environments. Of particular interest is the importance of gait among limbless animals. For example, does speed or efficiency motivate limbless animals to shift from slithering to sidewinding? To that end, we plan to develop models of the internal mechanics of the animals, which will be useful in determining their inherent metabolic and muscular limits.
To gain this broad understanding, we must develop force laws for both above and below ground that can accommodate a wider range of substrates. For which materials is our approximation of Coulomb friction a good one? What happens when an animal buries into wetted material? Can we use similar empirical forces laws, or if not, how are they modified? With improved models of environments and organisms, and their interactions, our approach can help find designs for future limbless robotic devices that can move through complex terrain faster and more efficiently than their natural counterparts.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.