Storied Theory
By Roald Hoffmann
Science and stories are not only compatible, they're inseparable, as shown by Einstein's classic 1905 paper on the photoelectric effect
Science and stories are not only compatible, they're inseparable, as shown by Einstein's classic 1905 paper on the photoelectric effect

DOI: 10.1511/2005.54.308
Science seems to be afraid of storytelling, perhaps because it associates narrative with long, untestable yarns. Stories are perceived as "just" literature. Worse, stories are not reducible to mathematics, so they are unlikely to impress our peers.
This fear is misplaced for two reasons. First, in paradigmatic science, hypotheses have to be crafted. What are alternative hypotheses but competing narratives? Invent them as fancifully as you can. Sure, they ought to avoid explicit violations of reality (such as light acting like a particle when everyone knows it's a wave?), but censor those stories lightly. There is time for experiment—by you or others—to discover which story holds up better.

The second reason not to fear a story is that human beings do science. A person must decide what molecule is made, what instrument built to measure what property. Yes, there are facts to begin with, facts to build on. But facts are mute. They generate neither the desire to understand, nor appeals for the patronage that science requires, nor the judgment to do A instead of B, nor the will to overcome a seemingly insuperable failure. Actions, small or large, are taken at a certain time by human beings—who are living out a story.
One might think that experiments are more sympathetic than theories to storytelling, because an experiment has a natural chronology and an overcoming of obstacles (see my article, "Narrative," in the July-August 2000 American Scientist). However, I think that narrative is indivisibly fused with the theoretical enterprise, for several reasons.
One, scientific theories are inherently explanatory. In mathematics it's fine to trace the consequences of changing assumptions just for the fun of it. In physics or chemistry, by contrast, one often constructs a theoretical framework to explain a strange experimental finding. In the act of explaining something, we shape a story. So C exists because A leads to B leads to C—and not D.
Two, theory is inventive. This statement is certainly true for chemistry, which today is more about synthesis than analysis and more about creation than discovery. As Anne Poduska, a graduate student in my group, pointed out to me, "theory has a greater opportunity to be fanciful, because you can make up molecules that don't (yet) exist."
Three, theory often provides a single account of how the world works—which is what a story is. In general, theoretical papers do not lay out several hypotheses. They take one and, using a set of mathematical mappings and proof techniques, trace out the consequences. Theories are world-making.
Finally, comparing theory with experiment provides a natural ending. There is a beginning to any theory—some facts, some hypotheses. After setting the stage, developing the readers' interest, engaging them in the fundamental conflict, there is the moment of (often experimental) truth: Will it work? And if that test of truth is not at hand, perhaps the future holds it.
The theorist who restates a problem without touching on an experimental result of some consequence, or who throws out too many unverifiable predictions, will lose credibility and, like a long-winded raconteur, the attention of his or her audience. Coming back to real ground after soaring on mathematical wings gives theory a narrative flow.
Let me analyze a theoretical paper to show how this storytelling imperative works. Not just any paper, but a classic appropriate to the centennial of Albert Einstein's great 1905 papers.
Einstein's paper on the photoelectric effect, published that fecund year, was singled out by the 1921 Nobel Committee (late as usual, and perhaps still afraid of relativity) as the basis for their award. It is also the only one of the 1905 papers that Einstein himself deemed revolutionary. But when one reads the article, the photoelectric effect appears late, as a denouement; the paper begins elsewhere.
The unwritten prologue is the contemporary interest in black-body radiation—the tendency of any object, no matter what its composition, to radiate light when it is heated. We see it in iron nestled in the forge, glowing red, then yellow, then white.
Chris Brodie
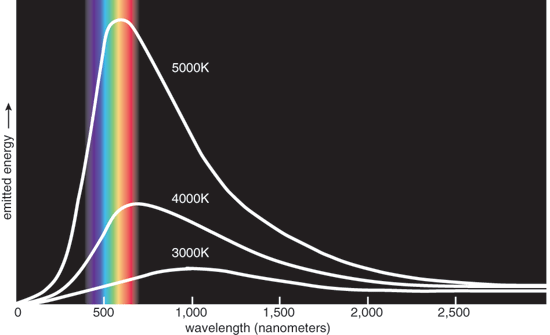
The intensity of this emitted light varies with the color (wavelength). At low temperatures, bodies radiate in the infrared. As the temperature rises, the maximum intensity of the radiated light moves into the red, then extends through the spectrum to the ultraviolet. At high temperatures, objects radiate intense light across the visible spectrum—that's white heat. The intensity of radiated light diminishes in the extreme ultraviolet and far infrared (see above). Astronomers estimate the temperatures of stars from just such curves.
The standard (and eminently successful) understanding of light in Einstein's day came from James Maxwell's electromagnetic theory. Coupled with thermodynamics and the kinetic theory of gases—a high expression of Newtonian mechanics—electromagnetic theory led to a "radiation law" that described how the intensity of light varied with wavelength at each temperature. The law fit the data—at long wavelengths. At short wavelengths, the equation derived from electromagnetic theory failed, in what became known as "the ultraviolet catastrophe."
In 1900, Max Planck found an expression that fit over the entire range of observations. Planck further perceived that his accurate radiation law could be obtained only if the energies of the little bits of oscillating charge that caused the light (he called them "resonators") assumed discontinuous values. So the quantum was born.
Planck had trouble believing that physics was, deep down, discontinuous. He spent many years searching for a way around what he discovered. But that is another story.
The photoelectric paper is modestly entitled, "On a Heuristic Point of View Concerning the Production and Transformation of Light." Einstein begins by stating the problem posed by the quantum hypothesis: He defines the resonators as bound electrons and takes us, with characteristic clarity, made possible by five years of experience with quanta, through Planck's derivation. He develops the characters in his tale—the radiation, Planck, his resonators, classical electromagnetic theory.

Then Einstein does something new. He sets out to derive Planck's radiation law without any assumptions about how light is generated. How does he do that? By assigning an entropy (the measure of randomness, a concept already in wide use by then) to the light and relating that entropy to the density of the radiation. Einstein proves that the entropy of the light in the black body varies with volume just the way that entropy varies with volume for that standby of freshman chemistry, the ideal gas.
This demonstration is direct. It's not Hemingway, but for scientific prose, really exciting. Einstein is taking us somewhere—we don't know where yet, but by the way he sets the scene, by his pace and conviction, we know something is going to happen.
Pretty incredible. No resonators, just a functional analogy of atoms or molecules to light. Playing out the analogy, light of a given wavelength could be described as if its energy came in dollops of what Einstein called Rβv/N, and today we would call hv, a constant (h) times the light's frequency (v). But that's just a way of looking at things—it's not for nothing that Einstein put the word heuristic in the title. Or is it? When do stories become real?
Back to the paper: Einstein has just rederived Planck's radiation law without resonators. Yet the discreteness of the light's energy, its quantization, is newly manifest in Einstein's work. There is no mistaking it. From this climax the paper cruises along another plateau, then swoops into a breathtaking shift of scene. Philipp Lenard had three years earlier observed "cathode rays," or beams of electrons, by shining light onto a metal. The phenomenon happened only when the frequency of that light exceeded a certain minimum; below that frequency (or above that wavelength)—nothing. After seeing the electrons, Lenard observed that their kinetic energy depended on the color of the light, their number on the intensity of the light.
This phenomenon we now call the photoelectric effect. Aside from being today a primary source of information on molecules and surfaces, the effect is behind photoelectric cells opening elevator doors, and is used in solar cells and light-sensitive diodes.
Back to 1905. Einstein just says: Let's assume light is quantized in units of hv, and that a "light quantum" (we would call it a photon today) gives up all its energy to a single electron. The electron needs a certain energy to leave the surface; if it has some left over, the extra contributes to its motion. Einstein calculates, in a couple of terse sentences, the energies involved and finds reasonable agreement with Lenard's measurements. With this and another calculation on the ionization of gases, he brings us down to experimental reality.
Except reality is not down, it is evidence. Evidence that this story of light being quantized is not just any story. This one is worth telling to our great-grandchildren.
Einstein's theory leaves us soaring, thinking what else this strange, discontinuous view of light might explain. Soon Bohr will use it to give us the first theory of an atom. This story is as exciting as Thomas Mann's 1902 Buddenbrooks, which Einstein might have been reading at the time.
The photoelectric paper was submitted to Annalen der Physik (Annals of Physics) in March 1905. But Planck's quantum theory, and the nature of light, had been on Einstein's mind for quite a while. On April 30, 1901 he wrote to his future wife, Mileva Maric, "I came recently on the idea that when light is generated, perhaps there occurs a direct conversion of kinetic energy to light. Because of the parallelism: motional energy of the molecules—absolute temperature—spectrum (energy of radiation in equilibrium). Who knows when a tunnel will be dug through these hard mountains!"
All theories tell a story. They have a beginning, in which people and ideas, models, molecules and governing equations take the stage. Their roles are defined; there is a puzzle to solve. Einstein sets his characters into motion so ingeniously, using entropy to tease out the parallels between moving molecules and the energy of light. The story develops; there are consequences of Einstein's approach. And at the end, his view of light as quantized and particular confronts the reality of the heretofore unexplained photoelectric effect. The postscripted future, of all else that can be understood and all new things that can be made, is implicit.
Perceptive reader Anne Poduska notes that the photoelectric paper "is particularly interesting because of the layering of perspectives (similar to legends being passed from one generation to the next, with each storyteller adding their own flair/details)." Indeed, Einstein uses Planck's development of the radiation law even as the younger physicist claims he will do it differently. He parlays belief in the discreteness of molecules (some of his contemporaries still doubted their existence) into an argument, first cautious, then growing in strength, of the discreteness of light.
A young man of 25, Einstein had mastered the old stories. In this paper he combined the ways others looked at the world, and trusting analogy as much as mathematics, made something new. Science is an inspired account of the struggle by human beings to understand the world. Changing it in the process. How could this be anything but a story?
Thanks to Anne Poduska for her careful reading and suggestions.
© Roald Hoffmann
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.