Obesity's Lasting Effect on Brain Signals
By Donovan Garner
Patients with obesity don't get the feeling that they are full, even after weight loss.
August 14, 2023
From The Staff Biology Medicine Anatomy
When Mireille Serlie has clinic hours in her role as an endocrinologist, she sees patients with obesity who tell her that they know they have eaten a big meal, but they don’t have any feeling of being full. Serlie, who has joint appointments at Amsterdam University Medical Center and Yale University School of Medicine, has taken that observation into her research work on obesity. As she and her colleagues have reported in the June 12 issue of Nature Metabolism, people with obesity appear to be correct: Their brains aren’t giving them the proper signals in response to nutrients. And what’s even more confounding is that when these patients lose significant weight, their brain responses do not change. “The brain really regulates food intake, and you would think that if there is enough caloric buffer in the body, that would then also make people stop eating, but apparently that’s not happening,” says Serlie.
Serlie and her colleagues began their study by figuring out how to bypass the brain’s response to the sight, smell, and taste of food, all of which are known to enhance brain activity in certain regions and trigger a release in the brain of dopamine, the “reward” chemical that helps people feel happy and motivated to eat. To study the gut-brain interaction, Serlie wanted to isolate the brain’s response to nutrients alone, so she and her colleagues used a nasogastric tube to infuse foods directly into patients' stomachs, stripping away the sensory experiences that usually accompany a meal. In separate trials, she and her team would either infuse lipids or glucose (both containing 500 kilocalories) into the gut. The study included about 30 people in each group, with obesity and of healthy weight, and the team used infusions of water as a control for brain responses to the feeling of volume alone in the stomach. The group also did not include participants who had childhood-onset obesity in an effort to try to reduce the inclusion of subjects with what's called monogenic obesity, in which the condition is the result of a mutation in a single gene that leads to the dysfunction of a specific protein.
After this infusion, the team measured brain activity in different regions using MRI to record what’s called a blood oxygen level-dependent (or BOLD) response, and they used what’s called single-photon emission computerized tomography (SPECT) to image each participant’s brain and detect dopamine release. When they looked at the scans of people with a healthy weight after infusion, their dopamine release would go up and their BOLD responses would go down in about 30 regions of the brain. In patients with obesity, glucose affected dopamine release as well but had no effect on the BOLD responses in their analysis of the whole brain. And direct infusions of lipids failed to trigger the dopamine boost or BOLD responses at all. This reaction didn’t change after patients with obesity had a weight loss of 10 percent of their original weight, even though their metabolic health improved in other ways. “Even though they lost a clinically significant amount of weight, the brain response to food in the stomach is not changing,” says Serlie. “That might actually explain why people have so much difficulty in keeping the lost weight off.”
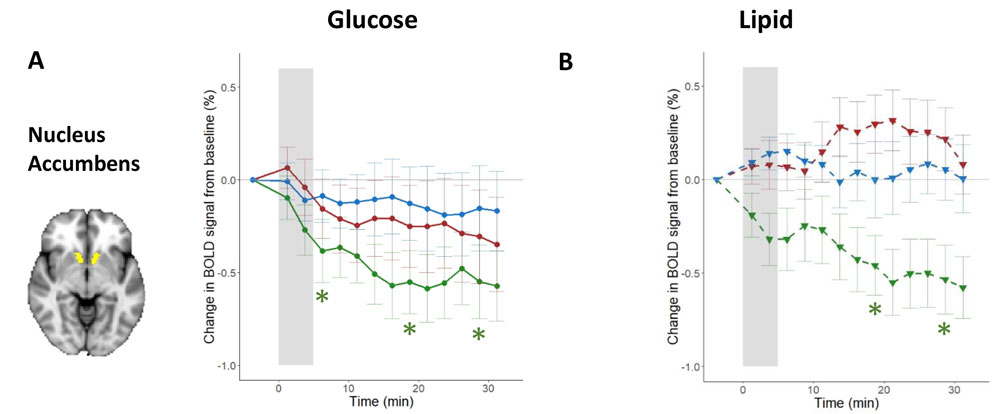
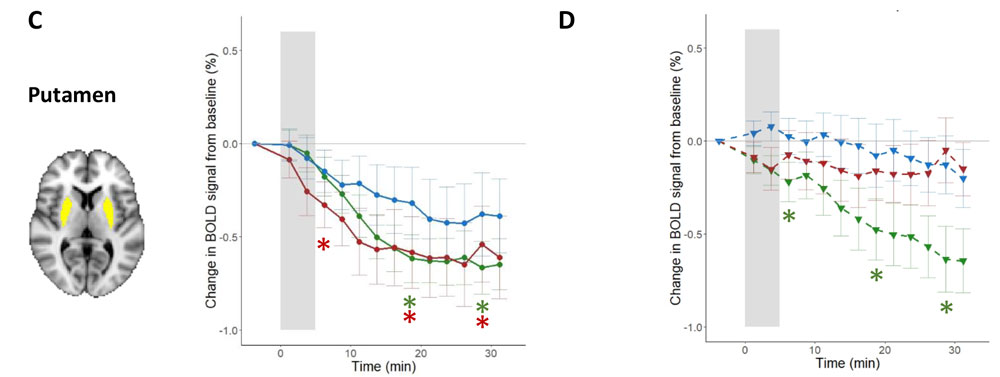
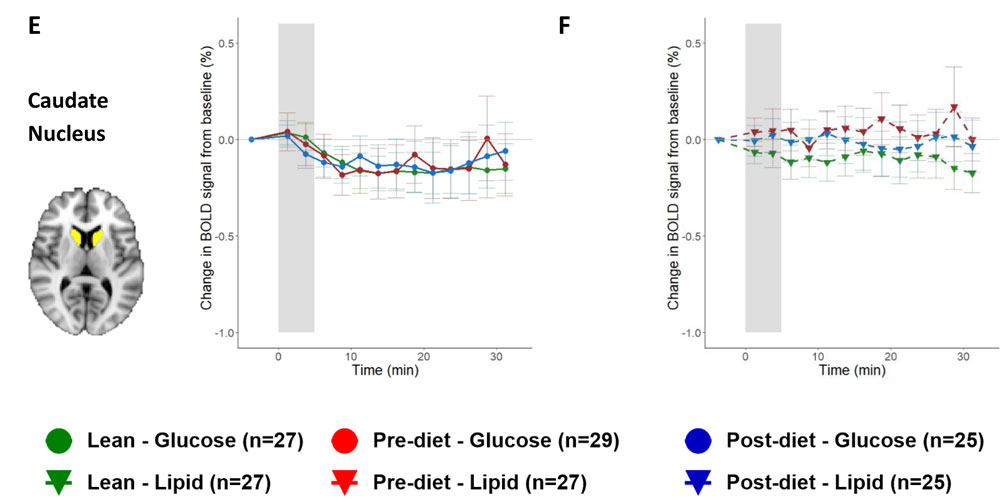
From K. A. van Galen, et al. 2023. Nature Metabolism DOI:10.1038/s42255-023-00816-9
Serlie hopes that more research might help to untangle reasons for the lack of food-induced dopamine response and response in other brain activity, including when this nutrient-sensing dysregulation begins to happen, the role of genetics, or the effects of different types of nutrients or eating patterns, and if there are any medications that increase nutrient sensing. Also, Serlie says longer-term studies are needed to assess the effect of weight loss on brain responses to food. The exact mechanism for how obesity, food, or eating patterns might be suppressing brain responses is still under investigation. And it remains unclear what mechanisms could explain the researchers’ observations so far. “The latter might be a miscommunication between the gut and the brain, and that could be related to the response of gut peptides or direct feedback through the vagal nerve, but it’s all speculation at this point,” says Serlie.
Although there is still a lot left to uncover, these results could have implications for how patients with obesity manage their consumption. Indeed, Serlie found that participants felt relieved and vindicated that they were correct that they were not getting brain responses to food. “Obesity has been recognized as a disease, but unfortunately, a lot of people, including doctors, still believe that obesity is a matter of choice, and losing weight is just a matter of eat less and move more,” says Serlie. “But although that's technically true, the reality is that it’s really difficult, and knowing that people with obesity cannot rely on the brain telling them that they ate enough might help. They could determine up front what they're going to eat and then they can stop eating even though they don’t feel like they are satiated. However, that’s so easy to say, but it might in fact help some people to understand why they would keep on eating.”
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.