Gutsy Engineering
By Robert Frederick
It takes bioinspired engineering just to study the cells lining the human gut.
June 14, 2018
From The Staff Biology Engineering Anatomy
It's mind-boggling how many places there still are to explore. I don't mean in space, the Solar System, or even on our planet. Just within the human body, many mysteries remain.
As you might imagine, one reason for this dearth of information is that postmortem studies contribute very little to our understanding of how healthy living tissues work, particularly with how those tissues interact with all the other organisms—bacteria, mostly—living in and on us. Scientists have only just begun to culture some of our own cells in environments that are at all similar to those inside each one of us.
For the cells lining the gut, however, it's still a challenge to culture them. In part, that's because they live on the boundary between the aerobic and anerobic: Our body's cells need oxygen, but oxygen is deadly to the gut bacteria that live in our guts and are nonetheless essential to our good health.
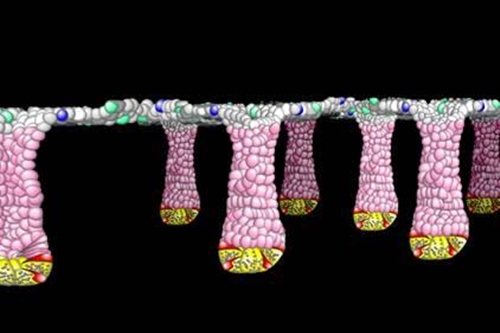
Image courtesy Scott Magness.
To take on that challenge, a couple of years ago Scott Magness and his colleagues in the joint department of biomedical engineering of the University of North Carolina – Chapel Hill and North Carolina State University applied for and received a high-risk, high-reward (read, "gutsy") grant from the National Institutes of Health to engineer, the authors wrote, "a functional, in vitro replica… of the human colonic epithelium and its associated microbiome."
Three years in to the five-year grant period, Magness and his team have developed an array of techniques and technologies to tackle the problem, and he gave an update on the project at a Research Triangle Park chapter meeting of Sigma Xi (the organization that publishes American Scientist).
Having asked a few follow-up questions, I made a short podcast about the bioinspired engineering Magness and his colleagues are doing just to study the cells lining the human gut. Their work has implications for how doctors could treat future cancer—using more chemotherapy and radiation—and in understanding how our diet and our microbiome affect our health.
A full transcript of the podcast is below.
[music]
Robert Frederick
If you get chemotherapy or radiation to treat cancer, the tissues in your gut are usually among the first to die, even if that’s not where the cancer is. That’s because those stem cells in your gut are particularly susceptible to that radiation and chemotherapy because they grow and divide so quickly. Of course, that allows them grow back quickly, too.
Scott Magness
And the genes that drive that process to regenerate your gut are unknown. We have no idea what happens there.
Robert Frederick
On this episode of the American Scientist podcast, the bioinspired engineering it takes just to study the lining of your gut. I’m Robert Frederick.
[music ends]
Robert Frederick
The cells lining your gut live about a week. And there are a lot of them. If you were to spread them out in a single layer, they would cover half of a tennis court.
Scott Magness
That is a lot of cells. And that’s all a stem-cell driven process.
Robert Frederick
Scott Magness is a biomedical engineer at the University of North Carolina - Chapel Hill.
Scott Magness
What that means is a stem cell is an undifferentiated cell. It doesn’t really know who it is in life yet. It can turn into a lot of different cell types of the gut, but it has to go through a number of genetic processes to do that, and we try to understand that.
Robert Frederick
The effort to understand those genetic processes is also an effort to understand what goes wrong.
Scott Magness
If it goes wrong, means you have a lot of problems. You have bacteria in your gut that get in your blood stream—you can die within hours if that happens—there’s inflammatory responses that can cause all kinds of problems that you know about.
Robert Frederick
Like diarrhea, bleeding ulcers, stomach pain, cramping, bloating, anemia.
Scott Magness
And then hopefully all of this information can help us develop cell-based therapies using stem-cell technologies, and also high-throughput screening platforms for drugs.
Robert Frederick
Particularly, Magness says, the kinds of drugs that would allow the gut to endure more aggressive cancer treatment.
Scott Magness
So if you want to dose somebody with higher chemo or radiation, you might be able to give them another drug that will enhance that regenerative process, so you can dose people with higher amounts for better outcomes. And that’s one of the limitations now of clinicians is they can’t give you a really high dose because it kills your normal cells.
Robert Frederick
So what’s known so far about this regenerative process?
Scott Magness
So, that event of that regeneration after chemotherapy or radiation probably happens from one or two cells. And the genes that drive that process to regenerate your gut are unknown. We have no idea what happens there. So we thought this might be a really nice way to start dissecting out from a single cell that’s left over after one of those therapies to figure out what genes it goes through, and those could be potential targets for new drugs.
Robert Frederick
And the problem, as Magness and many other researchers found out...?
Scott Magness
It’s very challenging to culture these cells to do anything with them, and also to apply those to any sort of real-world problems.
Robert Frederick
And that’s where the bioinspired engineering comes in, which really got going about 6 or 7 years ago. Before that...
Scott Magness
The technology was not there to even culture these cells. So my lab pioneered this in the United States... and the pressures that we would experience in the lab and the challenges forced us to develop new technologies that eventually led to these biomemetic tissue constructs—and all that means is the ability to develop a tissue construct outside of the body in a dish that mimics what your biology looks like, or the architecture of your gut.
Robert Frederick
So these cells -- these stem cells that grow to form the lining of your gut—these cells that develop really fast—they’re really hard for researchers to grow them outside of the body. Step one of three steps to study these cells was just to get them to grow at all. Turns out
Scott Magness
They grow in a three-dimensional jello-like substance we call matrigel. And they won’t grow flat on a plate....
Robert Frederick
And allowing them to grow in a blob, well, they turn into what’s called an “organoid”—basically a little mini-gut made of thousands of cells of several types, but shaped like a sphere—suspended in this jello-like substance.
Scott Magness
So this particular technology to be able to put a cell in a culture that will generate a tissue construct or sphere was very challenging and it really required a lot of biology to figure out what components to put in that culture, but also we recognized once you have that structure, how do you study it?
Robert Frederick
So after getting them to grow, step two in figuring out how to study these cells was to get them to grow in a way that you could study it -- because a little blob that’s that’s suspended in a jello-like structure -- well, looking through a standard microscope it’s going to be out of focus most of the time because the little blob of cells is growing in all three dimensions, not just the two dimensions it would be if it were flat on plate.
Scott Magness
And that was a real problem because you can’t follow them, you can’t count them, you can’t study them from day to day—it’s super challenging.
Robert Frederick
Of course, there are fancier three-dimensional imaging techniques, and the team tried those, too, with each three-dimensional image even of a fairly small number of these suspended cells taking about 10 hours to make.
Scott Magness
So you can imagine it’s not a very practical approach to studying the biology of single cells and understanding how they will develop into organoids.
Robert Frederick
Because remember, these cells grow and divide really, really fast. So Magness decided he needed help—a collaborator with the expertise to help him figure out how to take a single cell and study it at the single cell level, alive, and over time as it became an organoid, and in a way that they could study lots of them at the same time, quickly.
Scott Magness
And Nancy Allbritten is the chair of the biomedical engineering department at Chapel Hill and NC State—it’s a dual-department program—and her expertise is, at least one of them, is to microfabricate things. You tell her anything you want microfabricated and she can microfabricate—and what that means is making something big into something really small....
Robert Frederick
Scaled down, so that the small version looks like the big version. Together, they created a device that holds 15,000 cells, each in their own little numbered well, or compartment—with an address—to follow how these cells, each one in their own tiny well, develop over time. So finally, step three then in figuring out how to study these cells was to figure out how to follow the growth of 15,000 of them.
Scott Magness
Well, one of the big problems, especially if you have graduate students and post docs is how do you quantify 15,000 of anything. People will quit your lab if you tell them to count 15,000 of anything from day to day, from hour to hour. So we developed a computer-vision analysis platform that would tell us—let the computer tell us—what was in each one of those wells.
Robert Frederick
So the computer takes a picture of each one and then automatically analyzes each image for the circular shapes of cells.
Scott Magness
And so we had it look for circles and tell us if it was a circle of a certain size, ... and we found that this process was 99% accurate. So we could go through those 15,000 wells in about 2 hours and tell us with greater than 99% accuracy what was in those wells.
Robert Frederick
Like whether they had grown or divided. Finally, it was time to do some biology.
Scott Magness
You can look at the DNA, you can look at the genes, you can look at a lot of proteins, there are a lot of things you can look at...
Robert Frederick
Including testing the hypothesis that these stem cells grow better when they’re in close proximity—perhaps touching—other cells in the gut called paneth cells. Turns out that they do.
Scott Magness
That touching event was essential to confer survival and growth advantage of stem cells. Now how and why—what are the downstream things that are involved in that—we don’t know yet, and those are now a whole new field in our lab is to understand that process.
Robert Frederick
And in addition to looking for new pharmaceuticals to help these cells survive radiation and chemotherapy, other new research paths include questions about how all these cells interact with the bacteria that live inside your gut.
Scott Magness
There are thousands and thousands of different species and we do not know what they do. There’s people that will say that if you eat yogurt, that helps you out. We don’t really know exactly what that’s doing. There are -- people have inflammatory bowel diseases and it’s thought that there’s skewing of those thousands of different species in a way that you, your body, is attacking its own cells because it’s trying to fight off those bad bacteria in your body. Don’t understand that—we don’t get what bacteria are driving that and why some people are susceptible and some people are not. And the reason is ‘cause that we haven’t had tools to study it in a very detailed way.
Robert Frederick
Now they do... sort of—because there are a whole new set of challenges ahead in keeping human gut cells alive—they need oxygen—right next to and interacting with species of bacteria that are anerobic and would die in the presence of oxygen.
Scott Magness
So it’s still hard, ‘cause if one cell dies, you have a hole and the oxygen goes through.
Robert Frederick
And so with a grant from the federal government called a transformative R01—the kind of grant for a high-risk, high-reward idea—Magness and his colleagues are now three years into a five-year project to do just that.
Scott Magness
It’s really to develop these platforms that are biomemetic -- that are like you, but in a dish.
Robert Frederick
Scott Magness is a biomedical engineer at the University of North Carolina - Chapel Hill.
[music]
Robert Frederick
You’ve been listening to a podcast from American Scientist magazine, published by Sigma Xi, the Scientific Research Honor Society. I’m Robert Frederick. Thank you for joining us!
[music ends]
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.