Chasing Stardust
By Thanassis Psaltis
A quest to find sodium from stellar explosions can reveal more about how elements are made.
May 28, 2024
From The Staff Astronomy
If a new, bright object appears in the night sky, it might be a cosmic dance between two stars. These novae (meaning new in Latin) are actually violent stellar explosions. The “dance” takes place with an old, evolved star, called a white dwarf. This stellar remnant has very intense gravity because it is extremely dense: A white dwarf the mass of our Sun can be compressed into just the size of the Earth. Material from its dance partner, which can be a star like the Sun, is drawn towards the hot surface of the white dwarf. This material accumulates until the temperature reaches around 10 million degrees Celsius, and then a nuclear explosion happens. (A nova explosion differs from a supernova in that it doesn’t destroy the star.)
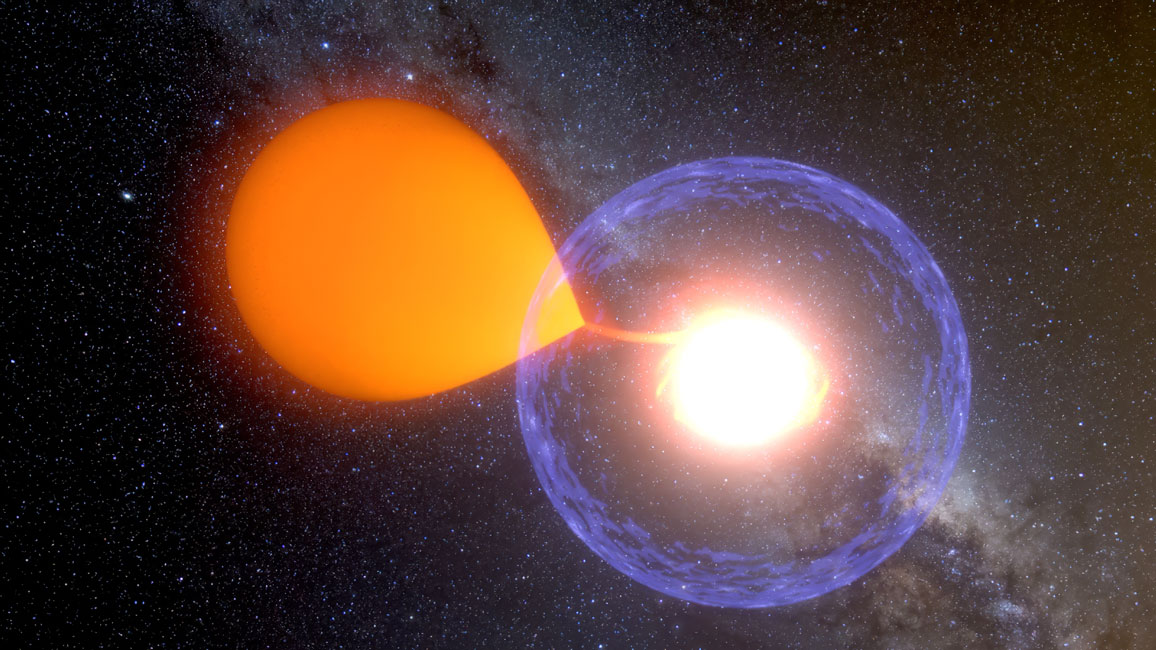
Image courtesy of K. Ulaczyk / Warsaw, University Observatory
Light from novae can help scientists explain how the chemical elements are produced in the cosmos. The extremely high temperature and density reached in the explosion allows the atoms of the deposited material to fuse into new elements that can be found on Earth, such as sulfur, lithium, and sodium.
One way to get a glimpse of the inner workings of novae explosions is by detecting their emitted light. Astronomers have managed to observe light that originates from the radioactive decay of aluminum-26 throughout the galaxy, some of which is coming from novae explosions.
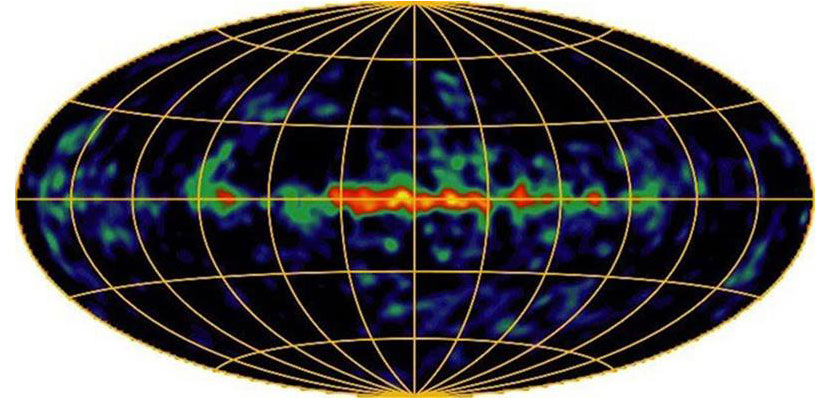
Image courtesy of the COMPTEL Collaboration
Theoretical astrophysicists have also predicted that novae create sodium-22, an isotope of one-half of the table salt molecule, which produces a unique signature that can be detected with gamma-ray telescopes. However, these gamma rays have not yet been detected by space-based telescopes, leading to decades of debate among nuclear physicists. The missing piece to determine how much sodium-22 can be found in a nova lies in measuring the decay of a nuclear state of magnesium-23, produced in novae by the fusion of sodium-22 with a proton. The main issue is that this decay lasts only an extremely short time: 100 trillion times faster than a blink of an eye!
Many experiments have been performed to detect this elusive decay, but they have produced conflicting results. So a team of researchers has tried taking a different measurement approach. As they reported in an article in Nature Communications, physicists Chloe Fougères and Francois de Oliveira Santos from France, along with more than 60 researchers from a dozen countries, found a way to get more precision from their data.
The group used an innovative experimental setup that allowed them to measure both the production time of this elusive state of magnesium-23 and its decay, hence extracting its lifetime. Fougères says that it was similar to "taking a picture of the survival of the [nuclear] states." The idea for the experiment was born in 2015 during a conference in Belgium: The researchers would combine a high-resolution gamma-ray detector system, called AGATA (for Advanced Gamma Tracking Array), with the VAMOS++ magnetic spectrometer, which will separate the different reaction products by mass, similar to how light is split into different wavelengths by a prism. AGATA is an experimental setup that is managed by a consortium of researchers from 13 European countries and has been used for experiments in laboratories all around Europe.
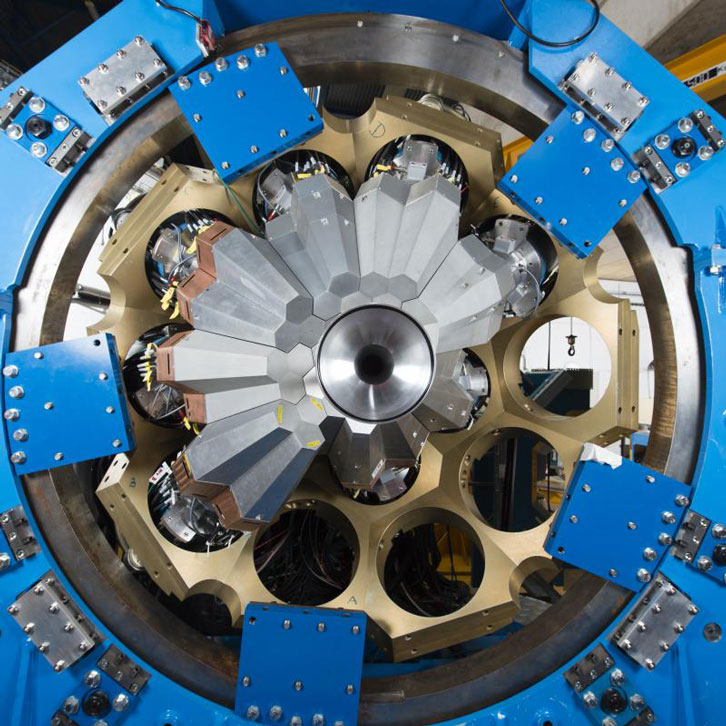
Image courtesy of AGATA, https://www.agata.org/
The experiment took place a year later at de Oliveira Santos’s and Fougères’s institution, the Large National Heavy Ion Accelerator, or GANIL, in Caen, about a three-hour drive from Paris. Unfortunately, the initial impression was that the experiment failed. "After two weeks of beamtime, running days and nights, and seeing mostly noise, it was quite depressing," says de Oliveira Santos. "At the end of the experiment, many people thought that we would not be able to get any results from the analysis, because of the noise."
However, this setback paved the way for something new. Fougères, a PhD student with de Oliveira Santos, began analyzing the data a year after the experiment took place, with a traditional technique that uses what’s called the Doppler effect (most people are more familiar with its acoustic counterpart, such as the change in pitch of a siren as it approaches and then moves away). The results were not promising, however. So Fougères, along with de Oliveira Santos, developed a new, more efficient technique that could provide definite results, even with the limited statistics of the experiment. Their technique works by calculating the difference between the velocity of magnesium-23 at the time of the reaction and the velocity after the emission of a gamma-ray. "This new method has many advantages and reduces the systematic uncertainties that traditional methods have," said Fougères.
This reduction of uncertainty allows for a more systematic study of the astrophysical conditions that can be found in a nova explosion. The group was thus able to perform simulations of novae explosions to predict the amount of sodium-22 that is produced. Using that simulation, they also calculated the maximum distance that the elusive sodium-22 gamma-rays from a nova explosion could be detected, which turned out to be around 3 kiloparsecs, roughly a third of the distance from Earth to the center of the Milky Way galaxy.
The next generation of space-based gamma-ray telescopes will be able to detect this unique signature from nova explosions, which will aid in the understanding of the physics of space explosions and the production of the chemical elements in the cosmos. Novae continue to occur and provide additional data for observation: As an avid amateur observational astronomer, de Oliveira Santos recently embarked on an observing tour for a nova that exploded in the Andromeda Galaxy.
"You have to never give up, even if it is hard,” said de Oliveira Santos. “In the end we got the results we wanted to get, and probably even more than that."
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.