The Myxomycetes: Nature’s Quick-Change Artists
By Harold W. Keller, Sydney E. Everhart, Courtney Marie Kilgore
Slime molds thrive in a range of environments, displaying an unexpected beauty in a variety of forms and life cycle stages.
Slime molds thrive in a range of environments, displaying an unexpected beauty in a variety of forms and life cycle stages.

And there it was: a jellylike mass creeping on the bark surface of a white oak tree some 25 meters high in the tree canopy, leaving behind a network of veinlike black tracks where it once was feeding. The trail of tracks eventually led to tiny fruiting bodies holding spores, which had formed hidden in the bark fissures. The spherical spore cases had a brilliant color combination of metallic bronze, shining silvery to glittering gold, and a bluish iridescent surface reminiscent of spectral rainbow colors. The spectacular display of multicolored iridescence contrasted with a pinkish stalk. Those traits, along with unique microscopic characteristics, indicated that this species was new to science. Later it was given the name Diachea arboricola H.W. Keller & M. Skrabal, because this discovery was made by an undergraduate student and climber, Melissa Skrabal. The species was one of many our team collected as part of a tree canopy biodiversity research study in Great Smoky Mountains National Park, which spans the border between Tennessee and North Carolina.
Before this discovery could be made, our research team had to get up into the high treetops. Our students had already completed a multiday climbing school, learning how to handle ropes and tie knots. They hiked several hours, backpacking with their ropes to our collection site, where they used a large slingshot attached to a pole—to launch a rope (weighted with what’s called a throwbag) over tree limbs some 20 meters high. They ascended and descended safely on ropes and harnesses, taking samples of bark every 3 meters. Over four field seasons, our students sampled more than 240 trees. At night, our team used flashlights to look for reflective fruiting bodies on the undersides of logs. We found 52 arboreal and 10 ground-dwelling species that had not previously been recorded in the park, bringing the total to 220 known species in that locale—including the one that was new to science.

Renato Cainelli
These life-forms are called myxomycetes, also known as plasmodial or acellular slime molds, and their classification has baffled scientists for more than 300 years, since the first citation of a myxomycete in 1654. Their life cycle transforms them from a fruiting body that releases spores, to microscopic amoebalike single-celled organisms called myxamoebae, to swarm cells swimming with flagella. These cells then can merge to form a plasmodium—a moving mass of protoplasm with multiple cell nuclei surrounded by a membrane—that eventually passes through a series of developmental stages to form a fruiting body. Masses of these spore-producing fruiting bodies may be microscopic—less than 100 micrometers—or they may be much more conspicuous, exceeding 50 centimeters in diameter.
These varied stages—some moving around and gobbling bacteria prey, others producing mushroomlike stalks and dispersing spores—created a controversial biological puzzle. The animallike stages were emphasized by some researchers who included them in the animal kingdom, whereas others emphasized the plantlike stages with inclusion in the plant kingdom, and still others considered myxomycetes as a third form of life and included them in the fungi kingdom. Modern molecular evidence, however, suggests that myxomycetes are rather in the protist kingdom, which is a hodgepodge of mostly simple organisms. The myxomycetes include more than 1,000 known species worldwide across five orders, 14 families, and 62 genera, with some species being spectacularly beautiful, and others, not so much.
Neither parasitic nor disease- causing in animals, these organisms are highly important contributors to soil ecology, and they also have played a role in research that ranges from robotics to cancer to mathematical modeling of group cooperative behavior (see “E Pluribus Unum,” Computing Science, January–February 1999).
The plasmodia, when cultured separately, have been used experimentally to study how cancer cells grow out of control, as well as to investigate how such cells migrate and induce angiogenesis during tumor growth (see “Multiscale Modeling in Biology,” March–April 2007). Novel chemical compounds have been isolated from fruiting bodies that could be useful in medicine. Experiments using a plasmodium in a maze with food at the exit resulted in the plasmodium migrating through the shortest possible route and minimum possible duration solution, suggesting that the slime mold could process external stimuli and exhibited a kind of “primitive intelligence.” (See “Slime Memories,” In the News, January– February 2013.) The myxamoebal stage is potentially immortal and can be grown in perpetuity, serving as a source for lifespan, aging, and longevity experiments. Myxomycete fruiting bodies could remediate environmental problems such as ground pollution because certain species concentrate highly toxic levels of zinc and other elements. Some species have even flown on the space shuttle as a model research system to test cellular responses to low gravity in outer space.
Angela R. Scarborough
Indeed, their seeming otherworldliness has made slime molds the object of science-fiction imagination for decades. The Blob was released in 1958 and starred Steve McQueen in his film debut. The movie’s plot centers on a meteorite that crashed on Earth and released a living mass that continues to spread, grow, and eat people. In the end, the blob was defeated by being transported to the Arctic, where it will remain frozen forever, as long as this region stays below freezing. Perhaps such sci-fi accounts are why in 1973 a large, slimy mass made national newspaper headlines when it was found in a backyard in a Dallas suburb. It continued to grow until the yellow “Texas blob” caused enough concern that the fire department hosed it apart. The fruiting bodies were finally identified as Fuligo septica, a common yellow slime mold that grows on wet mulch and plant debris (it’s known as the scrambled-egg slime mold or, less attractively, the dog vomit slime mold). This drama unfolded over a three-week period, during which time residents were alarmed and feared that it had become a threatening lawn disease or was from an alien source that had invaded the site.
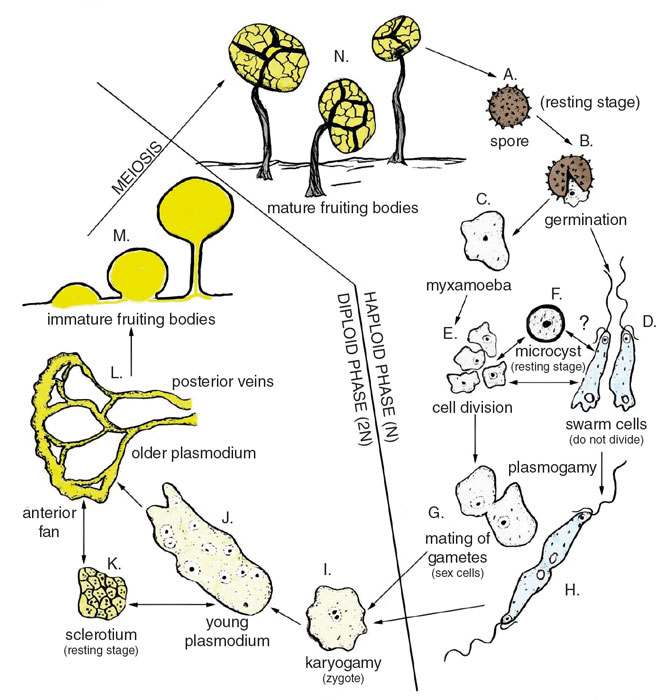
Throughout their lives, myxomycetes move through unicellular and multicellular stages that can look very different. A myxomycete’s fruiting bodies eject spores that result in unicellular gametes, which can grow into various multicellular forms. In poor growing conditions, a slime mold can go into a dormant stage as either a unicellular or multicellular organism.
Myxomycetes form spores inside fruiting bodies that open along a springlike hinge to release spores that are dispersed primarily by wind and secondarily by arthropods and rain droplets. Spores deposited in moist habitats germinate to produce one to four unicellular myxamoebae or swarm cells, each with one full set of chromosomes, making them sexual gametes. The myxamoebae are amorphous and surrounded by a plasma membrane that undergoes mitotic divisions, usually producing large numbers that crawl over its moist environment feeding on microorganisms, mostly bacteria. The swarm cell does not divide by mitosis, and typically has two whiplash flagella of unequal length at the anterior end. Mating may occur between two myxamoebae, a myxamoeba and swarm cell, or two swarm cells. The latter fuse only at their posterior ends, and often struggle to swim in a virtual tug of war during mating and fusion of nuclei. When free water is present, single swarm cells characteristically swim in a corkscrew rotational movement that is quite unlike most other motile aquatic microorganisms.
When exposed to unfavorable environmental conditions—such as drying, extreme temperatures, or toxic chemicals—the myxomycete cells can enter a dormant stage by forming microcysts when either the myxamoebae or swarm cells pull back into a rounded shape and develop into cells with protective walls. This encystment stage enables the myxomycete to survive for variable periods of time and then quickly revive to continue its life cycle when favorable conditions return.
After sexually compatible gametes fuse, the resulting zygote, now with two sets of chromosomes, begins feeding on microorganisms, including bacteria, yeasts, and other myxamoebae. The zygote gradually grows as its cells divide by mitosis to form a protoplasmic mass containing multiple nuclei—the slimy blob that gives these organisms their name.
These plasmodia vary in size, shape, and color, but are commonly bright yellow, white, or red. Ranging in size from microscopic to more than several meters in diameter, they are often conspicuous on decaying ground litter or logs. The largest plasmodia are visible to the unaided eye and typically have a broad, fanlike anterior feeding end and a posterior network of trailing vein-shaped material. These veins leave plasmodial tracks on leaves, wood, and bark, and are a good indicator that myxomycetes are present in the habitat.
The plasmodium can create pulsating movements using reversible protoplasmic streaming, the fastest known for any life form. The protoplasm flows toward the anterior feeding edge and then pauses, reverses, and flows toward the posterior end. The plasmodium is often encountered in wooded areas, creeping over the surface of decaying logs or leaf litter, or sometimes crawling up living plants to form fruiting bodies.
The plasmodium is capable of entering another resting stage, called a sclerotium, which is resistant to low and high temperatures, desiccation, lack of food, and toxic environmental and metabolic by-products. Sclerotia remain dormant during dry periods in the summertime on the bark of living trees and woody vines, and can remain viable for several years. In favorable conditions, they revive quickly: The active plasmodium crawls out and continues to feed, forming fruiting bodies in 12 to 48 hours.
The formation of fruiting bodies, also called sporangia, includes a series of gradual changes over time, beginning with the flattening of the plasmodium into a horizontal sheet that starts to form scattered nodules, usually along the veins. Protoplasm flows in and concentrates in these sporangial-forming centers, which gradually become raised pillars. Eventually the entire protoplasm of the plasmodium moves into individual fruiting bodies, forming a slender stalk with a globe-shaped head, where the spores eventually develop. Meiosis occurs inside the spores, dividing the sets of chromosomes and making the cells into gametes. Like all slime mold stages, the fruiting bodies can range in size from microscopic to readily visible. A mass exceeding 76 centimeters, discovered at the Botanical Research Institute of Texas in 2016, set the world record for the largest Fuligo septica fruiting body.
Immature fruiting bodies usually have the most brilliant colors, and thus are often collected in the field because they are easy to see and attract the attention of the curious naturalist. This immature soft stage, however, often dries out too quickly and becomes a hardened mass that is unidentifiable and not useful in a collection. Instead, pieces of ground litter or bark from living trees can be placed in a moist chamber and cultured in the laboratory, so developmental stages can be observed and harvested as viable specimens. Fully mature fruiting bodies of myxomycetes can be collected in the field on bark, wood, or leaves and glued in a small box for preservation in an herbarium. In this way, a permanent record can be maintained of fruiting body collections in a relatively small space. Spores that were stored in a collection for 75 years have been viable and still able to germinate.
The different colors, sizes, shapes, and structures of the fruiting body stage are key to identifying specimens and describing species. The most common fruiting body type is the stalked sporangium, which may have any combination of morphological characteristics: a hypothallus (the structure where the stalk joins the base), a peridium (the wall around the fruiting body head), and internally, a columella (the section of the stalk that continues inside the sporangium) and a capillitium (a network of threads inside the peridium), among other structures.

Kenneth L. Snell (left), Uno H. Eliasson (center, right).
For Diachea arboricola, the species our team found that was new to science, specimens collected on bark were cultured in the laboratory, and produced a bright yellow fan-shaped plasmodium stage before developing the glittering stalked sporangia. These sporangia included a number of unique features that helped to establish this new species. As the sporangia mature, the thin, iridescent peridium splits open to expose a whitish columella with branching capillitial threads attached at the apex. When the stalk is split open, it showed calcium carbonate crystals in rhombohedron shapes (see the figure above).
Iridescent colors are found in a broad diversity of animals and plants, and this visual flourish is present among the myxomycetes. The discovery of this species was an example of iridescent coloration among protists. Species descriptions indicate that iridescent colors occur in four of the five myxomycete orders (Liceales, Physarales, Stemonitales, and Trichiales).
Across species of Diachea, we have now found that the peridium likely has structures, rather than pigments, that impart the perceived iridescent and metallic colors. What appears to be a thin, transparent, smooth, and colorless peridium is much more complex when seen using scanning and transmission electron microscopes. The peridium surface is not uniform but has external bumps and roughness that contribute to variable thicknesses, and it has internal nanometer-scale air spacings of different sizes, all of which combine to create a multilayered inhomogeneous structure with irregular curvatures. The layers appear to consist of granules of dense material on a light background, with the external part of the peridium more translucent than the central part. Marina Inchaussandague and her colleagues at the University of Buenos Aires used a test optical model to measure the refractive index and incidence angle, and found that it accounts for the structural interference and iridescent colors. In collaboration with this group, we used fluorescence microscopy to show that in young sporangia, the peridium has more of an autofluorescent structure, in contrast with the iridescence that is more evident in mature sporangia. It is possible that this fluorescence protects the developing spores from damaging ultraviolet light.

Harold W. Keller (left), Robert Breshears (right)
Myxomycetes occur worldwide in terrestrial ecosystems—including forests, deserts, grasslands, and tundra, in order of most abundant species—and they occupy many different microhabitats on every continent. Freshwater ecosystems have yielded myxomycete fruiting bodies in moist chamber cultures but not as field-collected specimens. They apparently do not inhabit ocean ecosystems because they are intolerant of the salt content in marine water.
Species biodiversity usually increases with warmer temperatures, but the myxomycetes break this rule. The tropical rainforests have much higher tree species diversity, but the lowest myxomycete species diversity (although there are some species that are found only there). In contrast, temperate forested regions—including Great Smoky Mountains National Park, with its mixed conifer and broadleaf hardwood deciduous forest, and with stands of conifers at higher elevations—are biodiversity hotspots for myxomycetes.
Most of the known myxomycete species occur on or in decomposing forest floors. They can form fruiting bodies on leaf litter that accumulates in dry stream beds and ravines, under deciduous trees and shrubbery, from basal dead leaves in flower beds, and in leaf mulching and compost piles where leaves form deep layers and retain moisture over longer periods of time. Fruiting bodies may develop long after storm events in places where leaf litter is compacted into layers, and they will develop at the bottom of the layers next to the soil. Recent research suggests that if proper broad-based sequencing techniques are used that accurately detect myxamoebae, perhaps myxomycetes would be found to represent a dominant group of the soil microbiota.
Some plasmodia have a strong affinity for growing on or in decaying tree logs, stumps, woody branches and fragments on ground sites, and standing dead trees. This major group of species usually forms larger, more conspicuous fruiting bodies, and therefore are more frequently encountered and collected in the field.
Corticolous myxomycetes inhabit the bark of living, older, bigger trees and woody vines, so they are more frequently encountered and collected in the field, although it requires the use of a magnifying lens. These species apparently cannot compete well for space on ground sites and therefore have moved to where they have a developmental and survival advantage. The majority of these species have microscopic plasmodia; rapid sporulation usually occurring in less than 24 hours that produces a single, tiny sporangium less than 1 millimeter in height; and the quick release of spores via an evanescent peridium. These tiny stalked species are an example of a short-lived life-cycle strategy that can survive longer periods of unfavorable conditions, and then take advantage of brief periods of rain to complete their life cycle.
Outside of forests, a group of myxomycetes species do quite well in deserts and water-limited habitats, primarily growing on rotting pads and dried stems of cacti and succulents. The woody decaying remains of cacti stay moist over long periods of time and retain water from sporadic rain showers. Succulent plant tissues provide water and nourishment that stimulate plasmodial development and sporulation. Recent exploration of deserts and semiarid regions of South America, Mexico, and Russia have increased the numbers of new species that inhabit these areas to more than 70.
There are also some myxomycetes that specialize in the microhabitat of grasslands, especially herbaceous perennial plants with long floral stalks and dried fruits. These species adapt to higher summertime and colder wintertime temperatures, as well as drier and more alkaline pH conditions.

Renato Cainelli
And cold weather doesn’t stop the myxomycetes—indeed, some 98 obligate taxa thrive in mountainous terrain at the snowline, or underneath and along the margins of melting snowbanks. In winter, when the snowpack reaches a depth of 0.5 to 2 meters, the conditions create what’s called a subnivean zone: Latent heat from the ground melts a little of the snow and forms a thin layer of air, which is insulated by the deep snow and maintains temperatures near freezing. In this zone, the plasmodium can survive the subfreezing air temperatures and feed on a plethora of microbes also living in the soil. Fruiting occurs during spring in North America (from April into June) on forest litter and rocks, sometimes on stems of living shrubs or trunks of trees, or on the underside of prostrate evergreens such as junipers. As springtime air temperatures rise during the day, sometimes reaching 30 degrees Celsius, and the snowpack begins to melt, the plasmodia feed and grow until they eventually form large numbers of fruiting bodies—in some cases covering areas as extensive as 10 meters wide.
Many myxomycetes can show up in a range of locales (called facultative species). There are some species, however, that are restricted to special habitats (called obligate species). These same habitats are found in widely divergent locations in the world, suggesting that myxomycetes undergo adaptation to the physiochemical properties of the substratum such as pH, as well as other environmental parameters including temperature, rainfall, wind, and other species present. A few myxomycete species, for example, will produce fruiting bodies only on lichens, liverworts, or mosses on the bark of living trees or decaying logs. Others will grow directly on the surfaces of oyster mushrooms or other fungi, covering them with their bright yellow plasmodium. Still other species’ plasmodia will migrate long distances of about a meter from their point of origin on decaying logs, so they can form sporangia on living poison ivy, shrubs, and other herbaceous plants.

Renato Cainelli
One group of species live in a microhabitat created by the inflorescences of large plants found in the Central and South American tropics, such as Heliconia (often called lobster claws) and Costus (or spiral ginger). Rapidly decaying floral parts combined with a pH between 8 and 9 provide optimal conditions for these myxomycetes. Biologists Martin Schnittler and Steven Stephenson of Fairmont State University in West Virginia discovered this group of myxomycetes at study sites in Costa Rica, Ecuador, and Puerto Rico. It appears that this understory group of tropical plants provides an unfilled ecological niche that avoids competition from other ground-dwelling organisms while providing protection from tropical downpours that impede the colonization of more exposed habitats.
Animal dung creates another niche habitat for myxomycetes, housing at least 14 known obligate species. Although few are collected in the field, samples returned to the laboratory have been cultivated in moist chamber cultures to stimulate fruiting body development. Herbivorous dung sampled from grazing domesticated animals—cattle, horses, and goats—and from wild populations—bison and hares—produced myxomycetes. Certain species have greatly thickened spore walls that appear to be adapted to passage through the intestinal tract before germination occurs. However, the flip side of this protective wall thickening seems to be that sporangia lack a release mechanism, so how these species disperse their spores is a remaining puzzle.
Accidental or fortuitous occurrences can sometimes offer unusual habitats for myxomycetes. For instance, the stalked sporangia of the species Physarum pusillum have been observed all over the living cryptic lizard Corytophanes cristatus in a tropical forest of eastern Honduras. This lizard can remain motionless for long periods as it stalks insects, and this time (6 to 10 hours) must have been enough for the slow-moving, migrating plasmodium to eventually sporulate and cover the lizard’s body.
Myxomycetes can grow in association with green and blue-green algae (or cyanobacteria), forming fruiting bodies on wet rocks or logs that are covered in gelatinous layers produced by the cyanobacteria. The abundance and consistent presence of these microorganisms suggest that either they represent a source of food or provide other nutrients and associated bacteria. That could be why myxomycetes have sometimes cropped up in fish aquaria when algae is present. White plasmodia of the species Didymium iridis and Diderma effusum that were growing on the sides of glass aquaria were observed engulfing green algae, and that eventually resulted in unusually green plasmodia. In one case, the white plasmodium of D. effusum adhered to the head of a freshwater eel-like fish, Misgurnus anguillicaudatus, in an aquarium.

Anne-Marie Rantet-Poux/AMBAC
Myxomycetes may play an important role in the ecological cycle of many different habitats, but they also have a role in the food web. They are rampant consumers of bacteria, but they are also a food source themselves, and are often seen being consumed by insects or slugs. In a few places, some species are historically consumed by humans. The yellow plasmodium that took over the Texas backyard, Fuligo septica, is reportedly eaten by the native peoples from the area of Cofre de Perote in Veracruz, Mexico. This scrambled-egg-like stage is fried with onions and peppers and eaten wrapped in a tortilla. Another myxomycete, Reticularia lycoperdon, is known as the false puffball, and has been described as edible in its immature, mound-shaped fruiting body stage. People from the states of Veracruz and Tlaxcala, Mexico, call it the cheese mushroom and fry or bake it, saying it has a pleasant nutty taste. However, we hesitate to recommend any widespread foraging for myxomycetes, as many have not been tested for safe edibility.
The mechanisms by which myxomycetes in their unicellular phases join up to function as one multicellular mass have often been studied to see if they could help to untangle other cellular processes. For instance, Physarum polycephalum has been the myxomycete experimental organism of choice to answer basic questions of how cells maintain control or regulate growth during cell division. In its plasmodium, the mitotic synchronous cycle is apparently not triggered inside the nucleus but instead by a stimulator molecule that accumulates in the cytoplasm and is transferred to the nucleus shortly before mitosis. These results can be extrapolated and compared to unregulated cell divisions in which cellular internal control was lost, leading to the formation of cancerous tumors. Other experiments using plasmodia of Didymium iridis and Physarum cinereum aimed to determine lifespan and senescence, and showed that aging and longevity were under genetic nuclear control and not cytoplasmic factors.
Slime molds have also been an object of study for understanding self-organization and the evolution of cooperative behaviors. It was originally thought that there must be some kind of sentinel cells directing their group behaviors, but research and modeling have shown this step not to be accurate or necessary. When individuals start to coalesce, they release chemical signals that attract their nearest neighbors. Those in turn release their own chemical signals, creating a cascade effect that spontaneously forms large plasmodia. Transforming from there to fruiting bodies, some cells must become the stalk, and those cells do not get passed along as spores. Extensive research has investigated this sociobiological altruism, as well as methods for controlling “cheaters” who might try to get their cells disproportionately into spores. But if no cells become the stalk, no spores get dispersed, and everyone loses. (See “Evolution ‘For the Good of the Group',” September–October 2008.)
The ways in which slime molds move around have also been highly explored. For instance, the plasmodium of P. polycephalum has been used in the American, German, and Russian space programs to determine the effects of zero gravity on migration and protoplasmic streaming. Although the plasmodium survived under microgravity conditions, results showed that it had a clear sensitivity to gravity and light. These properties subsequently have been applied in a biorobot, in which a P. polycephalum plasmodium is the motive force that operates computer circuitry to directionally move lightweight floating objects and perform computations.
No myxomycete species cause a parasitic disease in humans. One way they can have negative impact, however, is with allergies. The windblown myxomycete spores of several species, including F. septica, have been recovered from mold spore air samples. Human subject clinical trial studies concluded that F. septica is an important aeroallergen and should be considered in the diagnosis and treatment of allergy patients.
But on balance, the substances discovered from myxomycetes have been beneficial. For instance, novel compounds isolated from 22 myxomycete fruiting bodies and plasmodia had a range of biological activity that included antibiotic, antimicrobial, and antiviral properties, as well as cytotoxicity to human cancer cells. None of these compounds have gone through human-subject clinical trials or received approval from the U.S. Food and Drug Administration. Nevertheless, the myxomycetes as a group have produced novel biochemical compounds that may someday prove to be useful biological agents in the treatment of human diseases.
In the meantime, myxomycetes may take an active role in helping remediate contaminated areas of the environment. Current research is studying how plasmodia of F. septica uptake and concentrate heavy metals, such as the hyperaccumulation of zinc. Plasmodia also take up lesser but still significant amounts of barium, cadmium, iron, manganese, and strontium.
Indeed, myxomycetes on bark could become indicator species as a biotic pollution index to monitor the health of trees, especially at higher elevations, as well as inland waterways, ponds, and lakes. Myxomycetes are sensitive to pH, which is affected by acid rain and air pollution. Laboratory cultures of bark from living tree species could be prepared and monitored for levels of myxomycetes plasmodia and fruiting bodies that would contribute to a better understanding of the health of nearby protected areas.
As more data accumulates on soil microorganisms, nutrients, and geochemical cycling, the importance of myxamoebae, swarm cells, and plasmodia in different soils will contribute to a better understanding of the ecological balance in the food web impacted by slime mold life-cycle stages and their role in maintaining a healthy soil microenvironment.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.