When Less Might Be More
By Felice Frankel
An aquatic protozoan yields its secrets under a polarized-light microscope
An aquatic protozoan yields its secrets under a polarized-light microscope

DOI: 10.1511/2006.59.262
When I first saw this color image, I couldn't figure out what microscopic technique was used to get those colors (which translate to information). I was pretty sure some sort of light polarization was used, but it didn't completely resemble the techniques with which I am familiar as an untrained user of optical microscopy. So I thought I'd invite myself to Danielle Cook France's lab. I was ultimately drawn more to a grayscale rendition from an aesthetic point of view, as I mention below. Perhaps I am dating myself. But no matter: I put myself in Danielle's capable hands, knowing there's no better way to spend time than learning from the next generation.
Danielle Cook France is a graduate student in biological engineering at the Massachusetts Institute of Technology. She holds a B.S. in biomedical engineering from Washington University in St. Louis. At MIT, she works with Paul Matsudaira, director of the Whitehead-MIT Bioimaging Center (http://bioimaging.wi.mit.edu). Her thesis work, which focuses on novel physical mechanisms of powering cellular motility and their applications to devices engineered from biological components, is supported by a Poitras Fellowship and the U.S. Army.
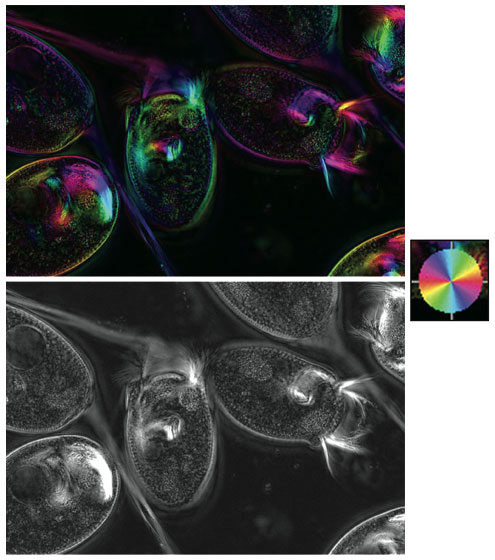
Courtesy of Danielle Cook France.
F. F. Danielle, can you describe to us how you produced the color-encoded image and what the colors mean? Why did you decide to use this particular technique rather than standard immuno-fluorescence imaging?
D. C. F. We know little about the proteins that make up the Vorticella stalk—the main calcium-binding proteins (centrin and spasmin) are known, but no other major structural proteins have been identified yet. This means that we exhausted our options for immunofluorescence imaging pretty quickly. Since TEM (transmission electron microscope) imaging from the 1970s showed the presence of small fibers, and I believe that centrin cannot form those fibers on its own, it made sense to go back to polarization microscopy to have a look at the fibrous structure.
I had seen a lecture by [microscopist] Shinya Inoué while I was taking the physiology summer course at the Marine Biological Laboratory at Woods Hole in 2004. I also got to see some of the microscopes in the lab, including the LCPolScope, which Rudolf Oldenbourg and collaborators at the MBL developed. Shinya used polarized light to see microtubules in dividing cells long before we knew what microtubules were, or that they were even there. Polarization microscopy has the ability to pick up a birefringence signal from biological fibers that are only nanometers in diameter. A lot of modern imaging has moved past that stage of initial discovery—the major components (like actin and microtubules) are already identified and easily tagged. But because I'm working on a relatively forgotten organism, polarization microscopy is very powerful.
The thing that Rudolf brought to Shinya's lab was the ability to generate orientation-independent retardance images. The LCPolScope uses liquid crystal polarizers to rapidly collect multiple images of the same specimen at different polarizations, or orientations. From those images, the maximum retardance at each pixel is calculated and stored in a new image (the black-and-white image shown was made from a two-frame technique, with the two frames captured at different orientations). The polarization direction in which that maximum occurred is also calculated, and can be overlaid on the black and white image as colorization. That's how we get the first color image shown.
F. F. I remember sitting with you at the computer and asking, just out of curiosity, what the image would look like without the coloring. When you showed the grayscale image to me, I was fascinated by how much more detail we saw. Do you agree? If so, why do you think that's the case?
D. C. F. Yes, I think that the black and white image is a little more striking. However, remember we are looking at two different images but with the same information in each. It's just that the black-and-white image shows the fibers within the stalk more clearly than the color image does; the color image shows that those fibers line up along the main axis of the stalk while it is extended. I could get all the alignment information from the color picture, but it's just a little easier to see distinct structures in black and white.
I don't have a rigorous optical/physiological explanation for it, but I think that we are better able to discern detail in the black and white picture because we better discern the contrast of white on black. The color picture adds another level of information which is helpful in a lot of ways, but does actually obscure some of the detail. I think you can see the same effect in photographs, and I think it's part of why Ansel Adams is so popular—he uses the full range of black and white to show all the immaculate detail that comes through in a landscape or a textured surface.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.