The Story of O
By Roald Hoffmann
Some surprising discoveries in oxygen chemistry and their meaning for our skies and cells
Some surprising discoveries in oxygen chemistry and their meaning for our skies and cells

DOI: 10.1511/2004.45.23
Everything in oxygen chemistry seemed more or less in place: Up there, in the stratosphere, there were oxygen atoms, O2 molecules and ozone, O3, as well as ions derived from these and a bit of active OH, all in a dance of creation and destruction.
Meanwhile, within our bodies, normal O2 served us well. There was even a place, under enzyme supervision, for the somewhat nasty relatives hydrogen peroxide (H2O2 and its deprotonated form, O22-) and superoxide (O2- and its protonated alter ego, HOO●), whose chemistry may generate the harmful hydroxyl radical ●OH. Of course, there's also water everywhere. And here and there singlet dioxygen, a more reactive and excited state of normal diatomic oxygen.
A nice, neatly compartmentalized world: ozone for atmospheric chemists, but not biologists, who had plenty of more complicated molecules to worry about.
So they thought.
This complacent state has now changed—dramatically so—with a series of remarkable discoveries. There is new evidence for the occurrence of ozone in living cells. Hydrogen peroxide is being made by molecules thought incapable of doing so. Even a metastable laboratory curiosity, the unusual HOOOH molecule (which sounds like a holler; call it dihydrogen-trioxide), may be in living systems. Meanwhile, we are still puzzling out the state of oxygen in high-temperature superconductors. And the menagerie of alternative forms of elemental oxygen continues to expand—there are strong theoretical arguments for the existence of a cyclic ozone isomer, and there may even be ways of stabilizing it.
Oxygen is the most abundant element in the crust of the Earth. It is mostly tied up in carbonates and phosphates, and in a wider range of silicates, from clays to zeolites to quartz.
Under ambient conditions, diatomic oxygen is the stable form of the element. O2 is a strange molecule, for all its ubiquity. Its problem (no, our problem as we try to think about it) is that oxygen's two least strongly held electrons, responsible for most of its chemistry, have available to them two orbitals. One electron goes into each orbital, so the ground state of O2 has two unpaired electrons.
From here on I will refer to this ground state simply as O2. The first excited state of O2, usually simply called singlet oxygen or 1O2, lies 95 kilojoules per mole above the ground state. Singlet oxygen is an energetic, still more reactive form of oxygen that is relatively easy to make, either chemically or with light.
Normal ground state O2 is not an inert molecule. A single unpaired electron on oxygen (as in ●OH) or on an organic fragment (as in methyl, ●CH3) makes such a "radical" very reactive. Radicals rip hydrogens from previously stable molecules; they also start polymerization chains. With its two unpaired electrons, O2 is a "diradical." It enters many organic and inorganic reactions—as we'll soon see, this changed the course of evolution.
But compared to other diradicals, O2 is surprisingly unreactive. "Otherwise California would be burning permanently, and not just from time to time all the time," as a La Jolla-based colleague remarked in the midst of the recent fires. Many organic molecules have large barriers to reaction with O2. The reasons for O2's attenuated reactivity are still being debated, but there is no ambiguity about the destructive nature of singlet oxygen and the ●OH and ●OOH radicals. Or of the metastable allotrope of oxygen, ozone. These attack, with alacrity, nearly anything organic.
Diatomic O2 apparently was not abundant early on in the history of the Earth, based in part on the age of oxidized iron deposits. So life, evolving as it did to utilize oxygen atoms in almost every molecule of consequence, got those atoms from carbon dioxide and carbonates. But the energy—and the electron donors and acceptors needed to run reduction-oxidation chemistry—came from elsewhere, likely first from reactions in the absence of light, such as H2 reacting with CO2. Later, with light but still without free O2, the energy and electron sources may have been bacterial photosynthesis, described by the schematic equation
2H2X + CO2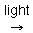 [CH2O] + 2X + H2O
[CH2O] + 2X + H2O
About two billion years ago, oxygen-releasing photosynthesis spread widely among biota, introducing vast amounts of O2 into the atmosphere. One might look at the 2X in the generic equation above as just a waste product. But nature is opportunistic. The leavings of one organism, even if dangerous, provide food for another. Or (for 2X= O2) a breathable gas.
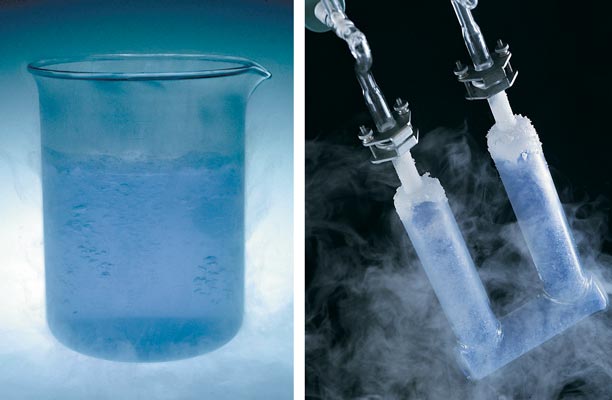
Yes, oxygen drove many of the early prokaryotes into anoxic niches, where they survive perfectly well to this day. Other survivors, prokaryotic and eukaryotic, evolved O2 detoxification strategies. These schemes included enzymes such as superoxide dismutase, classes of molecules such as luciferins, and some isoprenoids. With these and other molecular constables, aerobic organisms created an intracellular balance (perhaps upset as we age): Some oxygen-containing radicals were kept dangerous, even as aerobes evolved the biochemistry to control them.
And organisms did evolve to use O2. What was waste, now nourished. Yet oxygen does more than fuel cellular fires. The immune system is actively involved in making killing forms of oxygen, such as peroxide and ozone.
Part of the immune system is adaptive, an assortment of receptors that cover the surface of white blood cells. Some of these receptors are antibodies, which recognize and bind to pathogens. To actually destroy them, to disrupt bacterial membranes or initiate ingestion, immunologists thought that antibodies had to signal other parts of the system.
In the past three years, Scripps Research Institute chemists and biochemists have dramatically revised this picture. Richard Lerner, Albert Eschenmoser and Paul Wentworth, Jr., collaborating with several groups, first discovered that all antibodies could produce hydrogen peroxide. More recently, they have found that antibodies appear to catalyze the generation of ozone! The overall reaction (unbalanced, and obscuring many intermediate steps) is
H2O + 1O2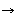 H2O3
H2O3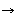 O3 + H2O2 + O2
O3 + H2O2 + O2
This reaction includes many of oxygen's protean molecular manifestations: Aside from the familiar water, hydrogen peroxide, excited singlet and normal ground state oxygen, it contains the unusual HOOOH molecule. Dihydrogen-trioxide was proposed more than 60 years ago, but direct proof for its existence has trickled in over the last 30 years. There is also a possible role for the HOOO● radical, not shown in the equation, which in turn may be a masked ●OH. All are reactive molecules, as shown by their short half-lives in water: around 1 minute for ozone, 20 milliseconds for HOOOH and 1 microsecond for 1O2.
The narrative of discovery that details the unraveling dogma is fascinating. Initial observations of H2O2 production in the presence of antibodies were startling enough. But as the Scripps group thought about what they saw, so much H2O2 was being produced that it became an enigma where all the electrons come from. The problem was resolved when the researchers realized that two molecules of singlet oxygen could react with water to give HOOOH, which could then go on to form ozone and hydrogen peroxide. A simple experiment ran the process in 18O-labeled water and showed that the label wound up in the H2O2; this result immediately supported the hypothesis. Albert Eschenmoser says:
The situation is reminiscent of photosynthesis when it started to use water as an electron donor and then began to poison the environment. It is "burning water," in one case by excitation of a cofactor by light, and in the other case by singlet oxygen mediated by an antibody.
And where does the singlet oxygen, the energy source, come from? Certain white blood cells produce it when stimulated by infection. In the tight economy of the cell (Lavoisier, the banker, would have loved this), everything is utilized.
Photograph courtesy of Paul Wentworth. Copyright 2000, National Academy of Sciences, U.S.A.
Recent work from the same imaginative collective has shown that atherosclerotic plaques also generate ozone. That ozone attacks cholesterol and the consequent oxidation products are all-around bad actors, implicated in cytotoxicity and mutagenesis.
This seemingly unorthodox chemistry has been with us (albeit unknowingly) for years. There is an old, potent oxidation system used to cleanse industrial quantities of soil and water contaminated with PCBs and worse, the peroxone process. In it, the samples are treated with a synergistic mixture of O3 and H2O2. In another cross-disciplinary study, the Scripps group, in collaboration with theoretician William A. Goddard, III, at California Institute of Technology, has shown that the peroxone process involves the same H2O3 molecule.
So now we have oxygen chemistry in the atmosphere, in the cell, in a decontamination process. But have we exhausted all of oxygen's secrets?
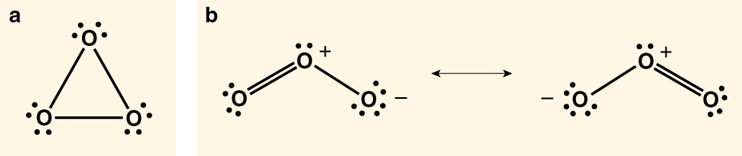
For a few intriguing small molecules you can draw a perfectly good Lewis structure. But they don't exist. Beginning chemistry students, nicely unsullied by the burden of knowing the answer, often give us one—ask them for a Lewis structure of ozone, O3, and they are as likely to write down the cyclic molecule in Figure 3a as the known form in Figure 3b, for which one has to draw two resonance structures. So what's wrong with the ring in Figure 3a? Or four-, or five-, or six-membered cyclic variants? The usual explanation is that the rings have too many unshared electron pairs close to one another—note the 12 dots, symbolizing electrons, in Figure 3a.
Tom Dunne
Now the situation grows interesting. A theoretical analysis shows that the interconversion of the normal bent form (the O-O-O angle in ozone is 117 degrees) and the cyclic form of O3 is what R. B. Woodward and I called a forbidden reaction. Which means that just the breaking of what one might have thought to be a single weak bond should have a substantial energy barrier. So there may yet be hope for stable cyclic O3.
Indeed, the best calculations today confirm the metastability of this ring. Cyclic ozone lies about 130 kilojoules per mole above normal O3 but has a barrier of no less than 95 kilojoules per mole preventing conversion to the open form. There is an even bigger barrier to falling apart to O2 + O.
Sulfur is like oxygen, in some of its chemistry. So what happens for the sulfur analogues of ozone? SO2 forms a three-sided ring that is at much higher energy (computed at about 400 kilojoules per mole) than the open structure (the geometries resemble those of ozone in Figures 3a and 3b). Nevertheless, cyclic SO2 also has a relatively large barrier to breaking a bond and opening up an angle—84 kilojoules per mole. S or O, there is no escape from the constraints quantum mechanics puts on reaction barriers. In S3 the ring is calculated to lie only 33 kilojoules per mole above the open form, again with a substantial barrier to opening.
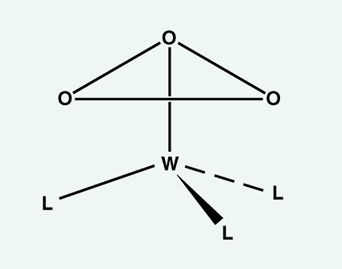
Cyclic O3 or S3 has not been detected. Should one give up on them? By no means. One of the beauties of organometallic chemistry is that an appropriately chosen MLn fragment (M=transition metal, L=ligand) can bind to and stabilize a molecule that, by itself, is unstable. So . . . to find the elusive cyclic ozone, one could try to make a molecule such as shown in Figure 4. Beate Flemmig, Shen-shu Sung and I are trying to predict what ligands on a metal (tungsten looks best) might be able to do this.
Actually, there has been one observation of cyclic ozone on an annealed magnesium-oxide surface. The technique used to image the molecules, transmission electron diffraction, showed O3 rings that appeared to be centered over underlying Mg ions.
Incidentally, the solid state is a fine place to look for oxygen in other weird forms. A mystery remains (one of many) about the chemical nature of oxygen in the cuprate superconductors. Oxygen doping is necessary for high-temperature superconductivity (high Tc) in systems such as La2CuO4. During the synthesis of this composite, the oxygen enters as O2, but it surely does not reach its equilibrium positions in the lattice in this form. To get at the problem in another way—all the signs are that the holes (missing electrons) in high Tc cuprates are partially on Cu2+, partially on (formal) O2-. But a hole, or one electron less, on O2- makes it O-. And this radical (here they are again!) will not sit still in the lattice. Large or small motions of the oxygens, static or dynamic formation of O21-, O22-, O23- or even a bigger aggregate—what will it be?
Would it surprise me to see cyclic ozone, or an as-yet unknown, metastable, oxygen-containing radical in a biological system? Two years ago I would have said, "You're crazy." After the beautiful and exciting Scripps work of the past two years, I'd rather leave the final word to Marie Anne Pierrette Paulze Lavoisier, who in a recent play about the element, puts it simply: "Imagine!"
© Roald Hoffmann
Thanks to Beate Flemmig for her help with some research on this article, Richard Lerner and Albert Eschenmoser for telling me of their work, and Lynn Margulis for enlarging my knowledge of microbial chemistry.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.