The Squeeze Is On
By Roald Hoffmann
How do molecules behave at extremely high pressure?
How do molecules behave at extremely high pressure?

DOI: 10.1511/2009.77.108
Scientists love subjecting matter to extreme conditions. And the variable of pressure, at its high end, is perhaps the most interesting one to explore for both chemistry and physics. For although we can estimate the (very short) lifetimes of molecules at temperatures of the sun, and what chemistry might transpire at a nanokelvin or in a vacuum “higher” than that of outer space, the realm of high pressure, such as that at the center of a planet, gives us pause.
The behavior of matter under high pressure is just not obvious, and this makes it fun to explore. No, it’s not sadism, just curiosity. Other motivations? It’s impossible to probe directly the core of Earth or Saturn; could we do it in the lab or on a computer? Also, predicting the behavior of matter under extreme conditions is a great test of whether we really do understand what’s going on.

Image is courtesy of Dr. Russell Hemley, Carnegie Institution of Washington.
Let me tell you about some remarkable goings on in the world of high pressure.
The international unit of pressure is the pascal (Pa); a common unit is the “bar,” close to the pressure of Earth’s atmosphere at sea level. 100,000 Pa make up a bar. The pressure in a tire is about 2.5 bar or so; the pressure under a high heel approaches 100 bar.
In the laboratory, pressures of a few hundred gigapascals (abbreviated GPa) are attainable. A pressure of 100 GPa equals 1 million bar (Mbar), or about 1 million atmospheres. The pressure at the center of the Earth is around 350 GPa, and this level is now within reach of a state-of-the-art experimental technique.
A typical piece of matter under 350 GPa of pressure undergoes a volume contraction by a factor of around 5 relative to its volume in ambient pressure. This means a diminution of every linear dimension of the piece of matter by a factor of around 1.7. Imagine squeezing a steel cube so that such a change happens; it’s a job not for the French cartoon strongman Obelix but for Diamond-Anvil Man.
Those researchers working near 350 GPa sometimes call the pressure range of 0 to 10 GPa a “near vacuum.”
To create such high pressures here on the surface of the Earth, it takes diamonds. A typical contemporary high-pressure “cell” consists of two diamond anvils and a bagel-shaped gasket that enclose a roughly 1 cubic millimeter reaction space (as shown in the first figure). Electrical leads can pass through that space, for heating or making various measurements. The diamonds are largely transparent, so monitoring by certain types of spectroscopy and x-ray diffraction is possible. A combination of hydrostatic and mechanical pressure is brought to bear on the diamond anvils; in the end, the highest pressure may be attained by a turn of a screw. A number of diamonds are lost in the process.
A small irony of matter works itself out in this apparatus. For diamonds themselves (natural diamonds, that is) were formed at high pressure deep in the ground, then brought up in pipes of kimberlite volcanic rock. Diamonds are also made synthetically under high pressure. They are thermodynamically unstable as compared to graphite, yet the barrier to that transformation is very high at ambient pressure. So once made, diamonds survive.
Will other, much stranger structures formed at high pressure also persist? So far, not many have. Many chemical reactions (for instance, the Nobel-prize-winning Haber-Bosch process for making ammonia from nitrogen and hydrogen, for use largely in fertilizers) are run under conditions of elevated pressure, typically a few hundred atmospheres, so as to maximize yields. However, really high-pressure science in the GPa range is not yet a synthetic procedure, except for making diamonds. That’s a problem for the trade—it would be nice to have a commercial raison d’être.
Seventy-five years ago, it was already foreseen that just about every substance will turn metallic under extreme pressure. Here is a list of the metallized so far:
• Xenon (Xe), a noble gas. But not (yet) any of the other noble gases.
• Iodine (I2), a molecular solid. As it approaches metallization, the diatomic bonds “dissolve” and the high-pressure structure features square sheets of iodine atoms.
• Not yet sodium chloride salt (NaCl), but cesium iodide (CsI) and barium telluride (BaTe), both of which are pretty ionic solids at ambient pressure.
The Holy Grail, for 75 years, has been hydrogen (H2). There are claims of metallization, but I think they are best characterized by the Scottish verdict “not proven.” Naturally, theoreticians have been very active in the area. One way to caricature the evolving knowledge is to say that every time the experimentalists reach the predicted pressure of hydrogen metallization, the theoreticians revise their estimate of the transition pressure up. What keeps people excited is that there is good reason to think that metallic hydrogen will be a high-temperature superconductor, and possibly a superfluid as well.
As the pressure rises, there is only one imperative: denser, denser. Two response strategies on the part of the matter in question are pretty obvious—the conversion of gases and liquids into solids, and the shift of any equilibrium that involves gases away from the side of the chemical equation that contains that state of matter.
Stephanie Freese
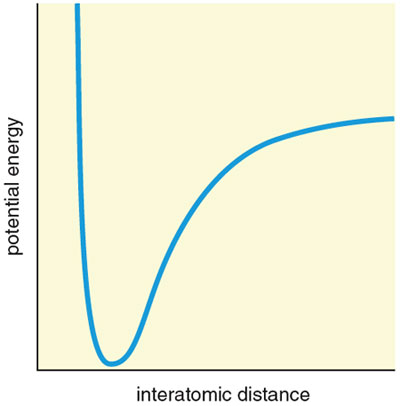
Squeeze some more. A molecular solid contains well-defined molecules with weak attractive forces (called dispersion or van der Waals forces) between them. A standard potential energy curve describes how the energy varies with distance between two such molecules (see the second figure, above).
Apply pressure, and the individual molecules come closer to each other. Actually, let’s think of a specific molecule, one my group has studied—silane (SiH4), the silicon analogue of methane. At ambient pressure the crystal has distances between nonbonded hydrogens of two different molecules of around 3.2 Ångströms. The analog computer that the molecule is itself tells us that this is the minimum of the potential energy curve—it’s as close as two hydrogen atoms of different SiH4 molecules wish to be.
As pressure is applied, the volume per SiH4 has to decrease. This can be accomplished by decreasing the distances between bonded silicon and hydrogens within each molecule, or by having neighboring molecules get closer to each other than they would at 1 atmosphere. Or both. Matter will do what hurts least, so to speak. It turns out that for SiH4 and most molecular solids we have studied, it’s the non-bonded intermolecular hydrogen distances that decrease, while the silicon-hydrogen distances remain pretty constant. “Van der Waals space” is squeezed out first.
Squeeze further. No choice—the atoms must get still closer together. It’s like sardines in a can, rush hour in the Tokyo subway. At some point the only way to take up less volume is to form more bonds—to increase coordination, to use chemical language. If you had four neighbors at one density, you may be forced to entertain six at a higher density.
Stephanie Freese
One of the most striking chemical consequences of high pressure may be seen for carbon dioxide (CO2) and nitrogen (N2). These are exceptionally stable molecules at ambient conditions, real thermodynamic sinks. Their analogues down groups 14 and 15 of the periodic table, such as silicon dioxide (SiO2) and phosphorus (P2), are also very stable thermodynamically (in fact, with respect to resisting decomposition into atoms, P2 is the most stable third-period diatomic). But they are not persistent kinetically, for waiting for them is the heaven of strong single P-P and Si-O bonds. SiO2 and P2 polymerize like a shot, giving us the many forms of silica and the several allotropes of P. Another way to say this is that multiple bonding is a good thing only for carbon, nitrogen and oxygen, but definitely not for their third-period or lower analogues.
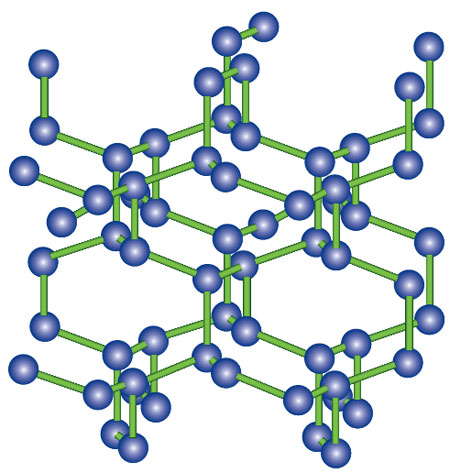
Here comes the surprise: Under only 12 GPa of pressure and temperatures of 1,000 kelvins (K), CO2 (already “close” to a solid at ambient conditions; witness “dry ice”) goes into a phase where the molecule is bent, but still molecular. At more than 35 GPa and 1,800 K, one gets a CO2 phase that resembles one of the forms of quartz, with not two C-O bonds, but four. At more than 110 GPa and 2,000 K, nitrogen polymerizes to a three-dimensional network resembling elemental P phases (as shown in the third figure, above).
These structure-alchemical transformations are driven by the desperate desire of molecules to compact and move to higher coordination.
As people discovered millennia before scientists began to think about it, the densest way to pack spheres (such as fruits) together is to make a hexagonal honeycomb layer, in which each sphere has six nearest neighbors, and then to stack such layers on top of each other. There are infinite ways to keep on doing this; in each, a sphere has 12 nearest neighbors.

Steve Hamblin/Alamy
The volume of space left empty in these closest packings turns out to be 29.6 percent of the overall volume, a fact long known, but proved mathematically only a decade ago.
There are many non-close-packed ways to arrange atoms or molecules. The gut feeling until a few years ago was that under pressure everything in the world would go into one of the two most regular close-packed structures, so-called hcp (hexagonal closest packing) or fcc (face-centered cubic, sometimes also called ccp).
Well, here’s what happens to elemental barium (Ba), as determined by the research of Richard J. Nelmes, Malcolm I. McMahon and their colleagues at the University of Edinburgh: At ambient pressure, Ba has a bcc structure (body-centered cubic, with 8 nearest neighbors for each Ba). At 5.5 GPa it goes fcc, and everyone is happy. But as the pressure is increased even further, Ba leaves the fcc formation for a seemingly less close-packed (yet denser) structure. This new arrangement is comprised of a “host” and a “guest” lattice (both made of Ba), which are incommensurate with each other. At still higher pressures, over 13 GPa, Ba falls into an incredible structure with nearly 300 atoms per unit cell.
In the past decade, nearly every alkali metal and alkaline earth metal structure has been found to go out of close-packing formations under an increase of pressure. Density rules. So how can you leave close packing? Easy. The following are some ways to think about it.
First of all, our prejudices are that atoms are spherical. And indeed spheres are limited to 70.4 percent packing efficiency. But who says atoms have to remain spherical as you push on them? Think about the extreme of cubes. They pack with 100 percent filling of space, right? Not that electron density in atoms will go cubical. But it can deform in that direction, or toward that of a number of other space-filling polyhedra.
Here is what Stephen Hales wrote in 1727, in his Vegetable Staticks (the quote is from H. S. M. Coxeter):
I compressed several fresh parcels of Pease in the same Pot, with a force equal to 1600, 800, and 400 pounds; in which Experiments, tho’ the Pease dilated, yet they did not raise the lever, because what they increased in bulk was, by the great incumbent weight, pressed into the interstices of the Pease, which they adequately filled up, being thereby formed into pretty regular Dodecahedrons.
Secondly, equal-diameter spheres do pack maximally to occupy 70.4 percent of space. But if you can have spheres of different sizes, you could put small spheres into the holes “between” the large ones, and so on. The problem of the efficiency of packing spheres of two or three unequal diameters has not been solved, to my knowledge. I bet that for a number of radius ratios one will get more dense packing than 70.4 percent.
So if you have an arrangement of equal atoms, and a limit to their packing if they remain equal, but a denser packing is available if they become unequal in size, perhaps they’ll do it. How can they become unequal? For instance, by shifting some electron density from one sublattice in the solid to another, a kind of electronic disproportionation, symbolized by ([A]n → [A+]n/2[A–]n/2).
A third factor: In a remarkable theoretical prediction, a few years ago my colleagues Neil W. Ashcroft and Jeffrey B. Neaton of Cornell University postulated that metallic lithium (Li) would move away from closest packing (it’s bcc at 1 atmosphere, fcc under higher pressure). Li atoms should pair up, and electron density moves into the crevices between Li pairs. With differences in detail, this risky prediction of a new mechanism for compaction was confirmed in 2000.
Atoms and molecules will do what they have to do to get denser, not what our simple minds tell them to do.
One of the interesting aspects of the field of structure under high pressure is that today the theoretical compression of a piece of matter is easier in the computer than in experiment. The software doesn’t fracture either, as the diamond anvils do from time to time. So the prediction of high pressure has become a theoretical playground.
Macmillan Publishers Ltd: Nature, 1999
But the problem of reliable prediction of molecular solid-state structure at ambient conditions is not solved. Some think this is a scandal. Actually, I’m happy it’s the way it is—room is left for intuition. Which older people retain.
The problem is that there are 230 space-groups (ways of arranging objects in 3 dimensions), and although many structures are simple, others show a very large number of atoms per repeat unit (recall that Ba structure). There are various methods for coming to the most stable structures—chemical and physical intuition based on bonding ideas and relationships to known structures, random searches in configurational space, and the use of evolutionary algorithms.
I mentioned our calculations on SiH4; a veritable industry of theoretical calculations that was spawned by our original paper (using the approaches mentioned above) has shown that the structures we suggested as most likely are in fact not those of lowest enthalpy. Others found better structures. We were wrong in detail; it’s OK, for from those imperfect geometrical optimizations we gleaned the general principles of what determines structure in the high-pressure regime, some of which I have described above.
I’ve given you just a glimpse of the rich chemistry found under high pressure. To a chemical intuition formed at terrestrial (surface) conditions, much of this extreme world seems strange. Actually, one can make some sense of it. And leave complete understanding for a (distant) future.
I would not have been able to write of this remarkable new chemistry and physics of high pressure without the inspiration and collaboration of Neil Ashcroft, Wojciech Grochala and Ji Feng. Thanks also to Russell Hemley and Richard Nelmes for their comments.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.