The Complex Call of the Carolina Chickadee
By Todd M. Freeberg, Jeffrey Lucas, Indrikis Krams
Can the chick-a-dee call provide lessons about language?
Can the chick-a-dee call provide lessons about language?

DOI: 10.1511/2012.98.398
If you live in North America, Europe or Asia near a forest, suburban open woodlands or even an urban city park, chances are you have heard a member of the avian family Paridae—the chickadees, tits and titmice. Birds use calls to communicate with their flockmates, and most parids share a unique call system, the chick-a-dee call. The call has multiple notes that are arranged in diverse ways. The resulting variation is extraordinary: The chick-a-dee call is one of the most complex signaling systems documented in nonhuman animal species.
Much research on the chick-a-dee call has considered Carolina chickadees, Poecile carolinensis, a species common in the southeastern United States. We focus on this species here, but we also compare findings from other parids. We discuss how the production and reception of these calls may be shaped over individual development, and also how ecological and evolutionary processes may affect call use. Finally, we raise some key questions that must be addressed to unravel some of the complexities of this intriguing signaling system. Increased understanding of the processes and pressures affecting chick-a-dee calls might tell us something important about what drives signaling complexity in animals, and it may also help us understand the evolution of that most complex vocal system, human language.
Toward the end of summer, many songbirds in temperate regions of the Northern Hemisphere migrate south to overwinter in more favorable climates. But some species stay put. One of the most common groups of resident songbirds is the chickadees and titmice of North America and the tits of Europe and Asia. These small songbirds (they typically weigh less than 30 grams) live in a wide range of habitats, often in heterospecific flocks—mixed-species groups that include other songbird and woodpecker species. Conspecific—composed of a single species—flocks of parids are often territorial and are reported to range in size from two (as in oak titmice, Baeolophus inornatus, which occur only as female-male pairs) to dozens of individuals (as in great tits, Parus major, which form large assemblages with fluid membership). Parids that form flocks do so in the late summer months and often remain in them until the following spring, when female-male pairs establish breeding territories. Such a flock structure, with stable groups of unrelated individuals, is atypical for songbirds and, as we argue below, may be an evolutionary force affecting vocal complexity in these species.

Illustration by Emma Skurnick.
Vocalizations in birds are often divided into two categories: songs and calls. Songs are typically given in the mating season and are directed toward mates or potential rivals. Calls are any other vocalization, and they fall into functional categories, such as food calls, contact calls, mobbing calls or alarm calls. In almost all songbirds, songs are complex and calls are simple. Not so with parids: Many species have relatively simple songs (for example, the fee bee song of black-capped chickadees, Poecile atricapillus, and the peter peter song of tufted titmice, Baeolophus bicolor ), but at least one very complex call system—the chick-a-dee call. The name “chickadee” for the North American Poecile group of parids is the onomatopoeic rendition of this call. Interestingly, it is labeled the si-tää call in willow tits, Poecile montanus, which are native to parts of Europe and Asia. When spoken in Swedish, Norwegian or Latvian, si-tää sounds quite similar to the birds’ call.
In winter months in many regions, the only bird sounds you may consistently hear are chick-a-dee calls. The source of those calls is likely to be a group of parids interacting with one another and with any number of other species of birds. Parids are commonly the nuclear species—the core members of mixed-species flocks; they are often joined for periods of time by satellite species such as nuthatches, kinglets, woodpeckers, goldcrests and treecreepers. The behavior of these nonparid species is affected by the presence or absence of parids and also by the parids’ chick-a-dee calls. As such, understanding social cohesion and group movement of these mixed-species flocks requires an understanding of parid signaling systems.
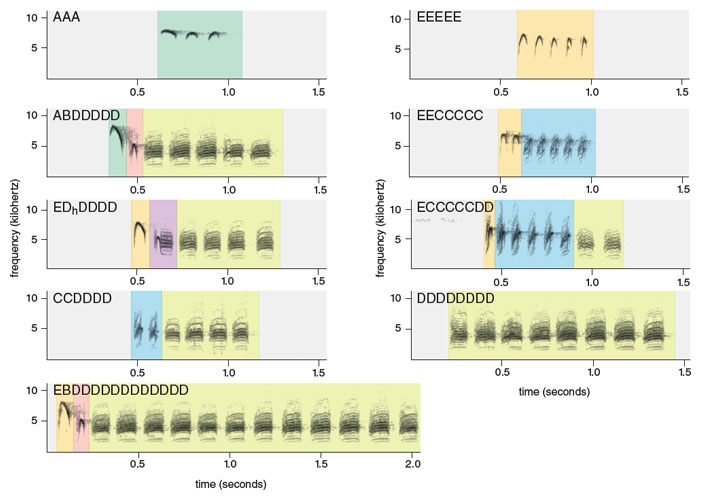
Chick-a-dee calls across parids share a number of acoustic features, each of which can be seen as somewhat analogous to aspects of human language. First, calls are composed of distinct note types. These note types have been categorized into acoustically distinct forms that can be distinguished by researchers with high reliability. In a 2012 study, two of us (Freeberg and Lucas) described six note types—A, E, B, C, Dh, and D notes—in the calls of Carolina chickadees from an eastern Tennessee population (see Figure 3). These note categories do not correspond to human musical notation; they are arbitrary labels. Christopher Sturdy and his colleagues at the University of Alberta have described a similar set of notes in the calls of Carolina chickadees and other chickadee species.
A, E and B notes are whistled and often show considerable frequency modulation. The C note is a noisy note type that generally increases in frequency over the course of the note. The D note, another noisy note type, has minimal frequency modulation. It seems to be a complex combination of two tones, or fundamental frequencies, and their harmonics, tones whose frequency is an integer multiple of the fundamental—along with other tones resulting from these tones’ interaction. (The songbird syrinx, or vocal organ, vibrates in two locations, one in each bronchus. Thus it can create two different tones simultaneously.) The final note type we described, the Dh or hybrid D note, is rare in this population and appears to be an A or B note that transitions without a break in sound into a concluding D note.

IPhotograph courtesy of Todd M. Freeberg.
Each note type normally occupies a specific part of the call. The typical chick-a-dee call in this population has an average of two introductory notes (some combination of A, E or B notes), roughly one C note, and three concluding D notes. Thus, the chick-a-dee call is made up of note types with distinct sounds, similar to the way each human language is made up of phonemes, or distinct sounds. (For example, the p and b sounds in English are distinct phonemes produced by the lips, called labial stop consonants; the difference between the two is that the b is voiced, or articulated by vibration of the vocal cords, and the p is not.)
Second, chick-a-dee calls are produced according to rules of note ordering. Roughly 99 percent of a sample of over 5,000 chick-a-dee calls followed the A– E–B–C–Dh–D ordering rule. Any note type can be repeated or left out of the sequence. So the chick-a-dee call has constraints on how the different sounds that make it up are combined to form calls, a phenomenon perhaps analogous to human-language constraints that govern how different phonemes are combined to form words.
A third commonality among chick-a-dee calls is that the call system is open-ended. The more chick-a-dee calls we record, the more calls with different note-type compositions are revealed. This variation is possible because notes can be repeated in calls, within the constraints of the note ordering rules. We know this from analysis, based on information theory (the study of the quantification of information, begun in the 1940s), of calls recorded from the Tennessee population we have studied. The phenomenon is also supported by within-individual analysis of chick-a-dee call note types derived from large sets of calls of known individuals recorded over time. This open-ended quality is one of the major differences between the chick-a-dee call and the finite call and song repertoires of most songbird species. Open-endedness is one of the defining features of human languages.
A final common characteristic among chick-a-dee calls is that they contain a large amount of information. In information theory, this term refers to the amount of uncertainty in a signaling system. When a signaler produces a signal, the information in that signal reduces the overall uncertainty to the receiver about the context of the signal—in other words, the receiver knows more about the signaler or the signaler’s likely behavior than it did before the signal was produced. Signaling systems with a large amount of information therefore can conceivably transmit a wide variety of distinct messages. The greater information content in chick-a-dee calls stems from the enormous diversity in their note-type composition. A key assumption of the concept of information as it is typically used by parid researchers (and other bioacoustics researchers) is that diversity of note composition relates to distinct messages in signals. Evidence from different labs and from different chickadee species indicates that the variation in chick-a-dee call structure documented via information-based analyses does indeed correspond to functional variation. Certain note-composition variants in these calls seem to be messages, often to flockmates, about the social and physical environment or the behavioral tendencies of the signaler.
Individual parids are often out of sight of flockmates as they move through the environment, so a vocal signaling system that can convey messages related to predators, food or group movement seems crucial to obtaining the benefits of group living. Recent studies indicate that variation in Carolina chickadee chick-a-dee calls is associated with these social and environmental contexts (see Figure 4). Chickadees and other parids have a number of distinct call types in their vocal repertoires, but our focus here is on chick-a-dee calls, so we use “calls” hereafter to refer to chick-a-dee calls.
Sound files courtesy of the authors. Spectrograms generated by the authors, using the Avisoft-SASLab Pro software application developed by Raimund Specht.
Most studies of these calls in the context of avian predators have used perched predators or models, as we along with Tatjana Krama and Cecilia Kullberg noted in a recently published review article. Christopher Zachau and Freeberg, in an article published this year, presented predator and control stimuli that “flew” in the area of Carolina chickadees visiting feeders. (See the sidebar on page 403 for more detail about the design of these experiments.) We used wooden models shaped like flying birds and painted to resemble either sharp-shinned hawks (Accipiter striatus, a threatening avian predator) or blue jays (Cyanocitta cristata, a nonthreatening avian control). The chickadees’ calls were recorded before and after the release and “flight” of the models down a zipline near the feeders. The calls produced varied with the presence of each model type, but the biggest effect we measured resulted from the flight of any model, irrespective of the species it mimicked. Calls produced after the model was released contained more A notes compared to calls produced prior to the release of the model. Greater production of A notes in the calls would seem to represent a message of alarm, as opposed to one of mobbing—behavior that is frequently linked to approaching and harassing predators—or of assembly. Tonal sounds that slowly increase in intensity and that are high frequency (such as the A note) are generally difficult for avian predators, and many other animals, to locate. In contrast, noisy sounds with rapid increases in intensity, like the D note, are easier to locate. Thus, the production of more A notes in these calls when a flying predator is detected in the area seems adaptive, as it could alert flockmates to the predator’s presence but not give away the location of the signaler to the predator.
Carolina chickadees produce more calls, and often more D notes in those calls, when they detect a perched avian-predator model than when no model is present. For example, in a 2009 study, Chad Soard and Gary Ritchison of Eastern Kentucky University placed six perched avian-predator models in the habitat of Carolina chickadees. The models, all of which represented hawk and owl species, ranged in size and type from small, agile predators like Eastern screech owls (Megascops asio) and sharp-shinned hawks to large, relatively slow-moving predators like great horned owls (Bubo virginianus) and red-tailed hawks (Buteo jamaicensis). The former predators represent real threats to small songbird species, whereas the latter do not. Chickadees produced more D notes in their calls when smaller, more threatening avian predators were present (see Figure 5). Later the researchers played back chick-a-dee calls recorded in these different threat contexts to chickadees in their habitat. The authors found that chickadees were more likely to mob the playback speaker—to approach it closely in large numbers—when it was playing calls recorded when a small predator model was present than when the speaker was playing calls recorded when a large predator model was present. This work suggests that easy-to-localize D notes are used more often in calls when those calls might serve a mobbing function—bringing flockmates to a particular location to drive a predator away. These findings make it clear that Carolina chickadees vary the note composition of their chick-a-dee calls in the high arousal contexts of predator detection and mobbing.
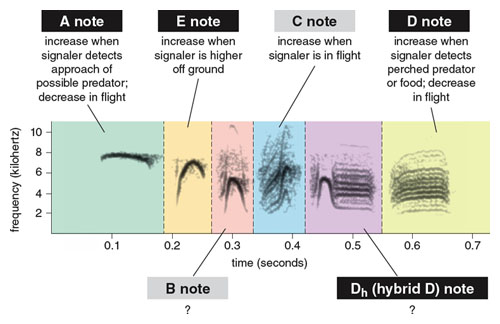
Illustration by Barbara Aulicino.
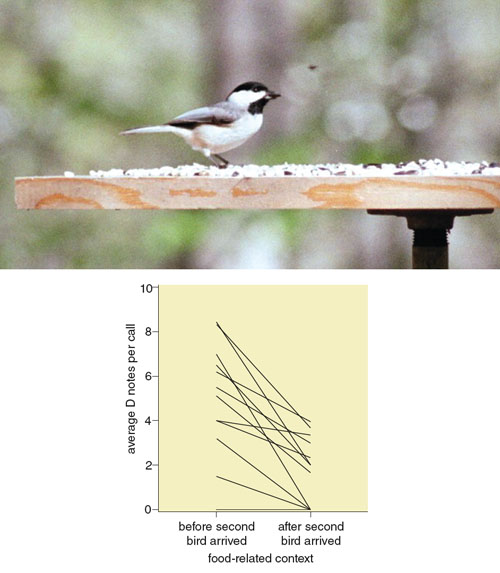
Ellen Mahurin and Freeberg found in a 2009 study that when individual chickadees from an eastern Tennessee population first detected food, the calls they produced contained a relatively large number of D notes (see Figure 6). Once at least one more chickadee arrived at a feeder, however, the first bird’s calls changed such that fewer D notes were produced. In a follow-up study near feeders at several sites, we played back calls that contained either a large number of D notes (which previous findings suggested might be a signal to assemble) or a small number of D notes (as a control). Carolina chickadees flew to and took seed from the feeders more quickly in response to calls containing a large number of D notes, supporting the notion that increased production of D notes can help recruit other individuals to the signaler’s location.
A naturalistic observation study conducted by Freeberg in 2008 suggests that chickadees use more C notes in their calls when they are in flight than when they are perched (see Figure 7). We have recently gained more experimental support for this suggestion: Chickadees flying to and from feeders produce calls with a greater number of C notes than they do when they are farther away from feeders. In addition, chickadees released from capture produce calls with a greater number of C notes when they are in flight than they do once they are perched. So calls with a relatively large number of C notes might signal movement—and thus might be adaptive for maintaining group cohesion in space.
In addition to these environmental and behavioral contexts, we have detected motivational influences on call production: Lucas, April Schraeder and Curt Jackson found in a 1999 study that chickadees increase rates of chick-a-dee calls when their energy stores decline. Additionally, there appear to be population-level “signatures” in the call that distinguish one population from another. There also appears to be marked variation at the individual level in call production. Evidence from Christopher Sturdy’s lab at the University of Alberta indicates that individual Carolina chickadees, as well as a number of other chickadee species, can often be statistically discriminated from one another by virtue of the acoustic characteristics of the note types of their calls.
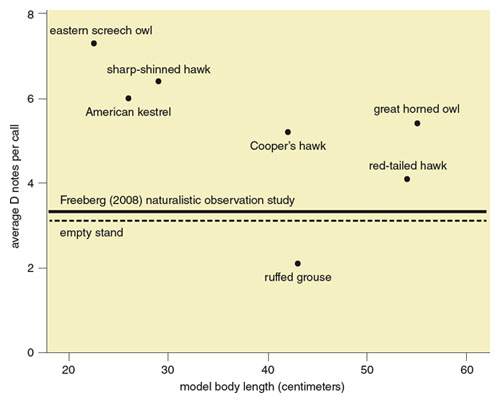
Figure adapted by Barbara Aulicino from C. M. Soard and G. Ritchison. 2009. Animal Behaviour 78:1447–1453. With permission from Elsevier.
We thus have considerable evidence that the note composition of calls of Carolina chickadees is associated with detection of predators (both perched and flying), food detection, individual flight and motivation. The calls also vary in ways that may suggest markers for individual, flock, population or some combination of the three. Variation in the note types that make up the call corresponds to different contexts and to population-level characteristics. Studies of call variation have also been carried out in other parid species. For example, as a 2012 review article by Krams and coauthors reveals, perched predator contexts have been shown to have a similar effect on call note composition in black-capped chickadees, Mexican chickadees (Poecile sclateri) and willow tits. Call variation seems to be associated with food contexts in black-capped chickadees and with flight contexts in mountain chickadees (P. gambeli). Krama, Krams and Kristine Igaune in 2008 documented variation in the comparable call system in crested tits (Lophophanes cristatus), based on whether individuals were close to the relative safety of vegetation or were exposed in open areas away from cover. Another interesting finding about this species is that dominant individuals use their calls differently than subordinate individuals, which suggests possible personality-like influences on call variation.
Decades ago the Dutch ethologist Niko Tinbergen described four different “why” questions researchers could ask in trying to understand the behavior they observed in animals. Two of the questions entail proximate approaches that focus on the individual. One of these proximate approaches includes mechanistic questions—what is the neural and physiological basis of the behavior, and what stimuli in the environment elicit behavior? The other proximate approach covers developmental questions—what roles do growth and experience play in shaping and constraining behavior over an individual’s lifetime? The final two questions are ultimate approaches with a population- or species-level focus. These are ecological or functional questions about the adaptiveness of the behavior—what problem might it have evolved in response to?—and they pose phylogenetic or deep-evolutionary questions—how might common ancestry shape and constrain behavior over the existence of a clade? We can use these approaches to help understand the chick-a-dee call.

Illustration by Emma Skurnick.
At a proximate level of analysis, we know that certain environmental stimuli or motivational influences generate variation in calls. In addition, the complexity of social groups in Carolina chickadees can drive complexity in the note composition of calls. In a 2006 study by Freeberg, chickadees placed into large captive flocks used calls with greater information content compared to chickadees placed into small captive flocks, suggesting that the diversity of messages is greater in more complex social groups. These experimental changes to the social groups of chickadees must have generated neural and physiological changes in the individuals in the study, yet we know relatively little about this aspect of the call. Sturdy’s laboratory has carried out a number of exciting studies related to the perception and discrimination of calls in individuals. Female black-capped chickadees reared in isolation fail to develop the ability to perceive relative pitch of males’ songs. However, we know relatively little about the ontogeny of call variation in young parids interacting with parents and, later, with nonrelated adults in their social groups. More work on proximate questions related to call variation is needed.
Photograph courtesy of Todd M. Freeberg. Graph data from E. J. Mahurin and T. M. Freeberg, 2009.
At an ultimate level of analysis, we can infer that the call is homologous across many different parid species, suggesting a fundamentally comparable call system in common ancestors to today’s chickadees, tits and titmice. We know a fair amount about call variation in a few species, but the calls of most parid species have been little studied, let alone the question of whether call variation corresponds to different environmental or behavioral contexts. As a result, we cannot yet answer many fairly basic questions about the evolution of call variation. At the functional level, we can infer that the call is adaptive in bringing about social cohesion in parid species, because variation in the call can recruit, alarm or potentially signal movement for members of both conspecific and heterospecific flocks. Whether variation in signaling with the call is related to differences in survival or reproduction is an open question. Nonetheless, a number of hypotheses have been proposed to explain the adaptive significance of call variation in parids.
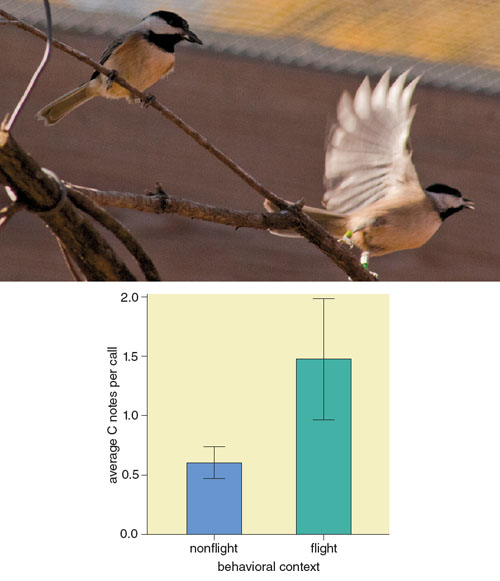
Photograph courtesy of Amy O’Hatnick. Graph data from T. M. Freeberg, 2008.
First, the complexity of the social group might influence vocal complexity. This argument is known as the social complexity hypothesis for communicative complexity, and it is supported by findings from a range of mammals, birds and nonavian reptiles, and from auditory, chemical and visual modalities. For the chick-a-dee call, the social complexity hypothesis predicts that populations in which individuals occur in larger groups or in groups with greater network complexity will have more complex calling behavior than populations in which individuals occur in smaller groups or in groups with little network complexity. If future research supports this hypothesis, we will be able to infer that social pressures that arise from interacting with the same individuals over time, in both competitive and cooperative contexts, require a flexible and diverse repertoire of signals. If the complexity of an individual’s social group impacts the diversity of vocal signals used in social interaction, that social group can be seen as both a context for vocal development and a potential selective pressure that could impact vocal behavior.

Illustration by Emma Skurnick.
Selection for increased signaling complexity in parids may also come from other species in mixed-species flocks. For example, Mark Nolen and Lucas found in a 2009 study that both white-breasted nuthatches (Sitta carolinensis) and tufted titmice interact vocally with Carolina chickadees when mobbing predators. The primary vocal signal used by chickadees under these conditions is the chick-a-dee call. Moreover, Chris Templeton and Erick Greene of the University of Montana in 2007 suggested that nuthatches can decode information about predation risk from calls, and recently Stacia Hetrick and Kathryn Sieving of the University of Florida found that chickadees can decode information about predation risk from the chick-a-dee calls of tufted titmice. These findings show that a complex call provides relatively fine-scale information about predation risk to conspecifics and heterospecifics. Both types of association have fitness consequences. The complexity of conspecific and mixed-species flocks may therefore drive the diversity and complexity of vocal signaling systems.

Photograph courtesy of Jorma Tenovuo.
Another hypothesis proposed to explain call complexity is the predation pressure hypothesis, which has support from a number of studies in primate species. It predicts that populations facing intense predation pressure or a variety of predator types should have more complex calling behavior than populations facing relatively light predator pressure. This hypothesis, then, would predict that parid populations or species that face a large number of different predators have a more complex call than parid populations or species that occur in areas with few predators. One more hypothesis to consider for call complexity relates to the physical environment in which individuals live. Parid populations or species living in complex physical environments, such as those containing a mix of open, closed and edge habitat, may require more complex calls to communicate effectively, compared to populations or species living in relatively simple physical habitats, such as exclusively coniferous forests. These three hypotheses (and there are others) may each explain the complexity and variation in chick-a-dee calls that we see. Perhaps our biggest need in answering this question is for large comparative data sets from multiple populations or multiple species, with which to test the various hypotheses.
We have discussed sociality in parids in light of the benefits of grouping, but we would be remiss if we did not point out that grouping also brings costs. Foraging in a group reduces energetic costs—individuals have more time to find and process food because they can spend less time detecting predators. But flocking also results in increased competition for resources and may generate higher stress levels. It may also increase transmission of and reduce resistance to parasites and pathogens. More work on the costs of grouping in parids should shed considerable light on the pressures individuals and their signaling systems face in complex social groups.
The Paridae family seems ideal for testing hypotheses for communicative complexity. As Jan Ekman of Uppsala Universitet pointed out in a 1989 study, it has considerable variation across species in key social dimensions such as group size, presence and number of heterospecifics in mixed-species flocks, and presence or absence of winter territories. For example, flocks in great tits (Parus major) are reported to range from 2 to roughly 50 individuals (see Figure 9). It is hard to determine flock size in this species, however, because great tits do not have a stable flock structure over time (individuals often move in and out of groups) or space (their flocks, unlike those of many other parids, are not territorial). Recent advances in assessing social networks in animal groups should prove important to determining social complexity in this species. We believe great tits could be a key species for testing functional hypotheses regarding call complexity.
Does the variation in social complexity we have been describing here explain variation in the structure and use of chick-a-dee calls? This straightforward question, like the questions raised by other hypotheses, remains unanswered simply because social and vocal behavioral data are needed for a greater number of parids than have been studied to date. For example, we know very little about the vocal behavior and social structure of African parids in the species-rich Melaniparus group, or of South and East Asian parids.
One example has been documented thus far of commonly occurring reversals of note ordering rules (where, for example, calls have both a note type 1–note type 2 order, and a note type 2–note type 1 order): In 1994, Jack Hailman of the University of Wisconsin documented this variation in the call of the black-lored tit, Parus xanthogenys, of India. The finding is an exciting and potentially important one: Vocal flexibility of this kind would greatly increase call complexity, and it has the potential to increase the variety of meaning receivers could obtain from calls. Such ability might also bring the call closer to the notion of syntax in human language—in which, for instance, “the child spoke to the toy” has a very different meaning than “the toy spoke to the child.” However, we can say very little about the potential pressures influencing the call system of the black-lored tit because so little is known about its social behavior or about closely related species in this geographical area.
We hope that this article will inspire increased efforts at understanding the social and vocal behavior of parids—such understanding is needed to determine the evolution of signaling complexity in these species. Furthermore, greater knowledge of the pressures shaping the chick-a-dee call system just might tell us a little more about the pressures that shape and constrain our own complex vocal system.
We thank Harriet Bowden, Sheri Browing, Gordon Burghardt, Esteban Fernandez-Juricic, Megan Gall, Jessica Owens, Kelly Ronald and Luke Tyrell for helpful comments on earlier drafts of this article. Todd Freeberg thanks the J. William Fulbright Scholarship Board for a teaching award in Latvia in the spring of 2012, which helped make the writing and preparation of this article possible.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.