Arsenic, the 'King of Poisons,' in Food and Water
By Andrew Yosim, Kathryn Bailey, Rebecca C. Fry
Levels of this poisonous element can far exceed the US Environmental Protection Agency's water standards in common foods such as rice.
Levels of this poisonous element can far exceed the US Environmental Protection Agency's water standards in common foods such as rice.

DOI: 10.1511/2015.112.34
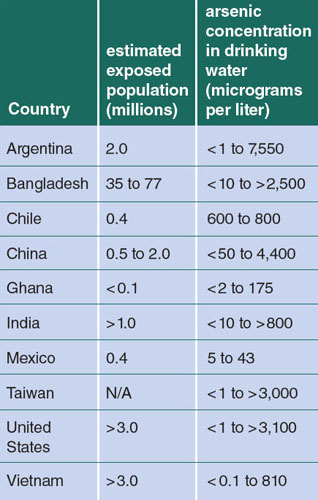
Arsenic is contaminating water in regions around the world, including the United States. For example, our recent work has highlighted that arsenic is contaminating the water of residents of North Carolina and Mexico with detrimental health impacts, particularly in children. The World Health Organization has called arsenic contamination in Bangladesh, with pervasive arsenic-rich drinking water resulting from tube well establishment, “the largest mass poisoning of a population in history.”

At present, it is estimated that more than 100 million individuals worldwide are chronically exposed to levels of inorganic arsenic in drinking water that can pose a significant threat to human health. Developing fetuses and children are particularly susceptible to arsenic; exposures have been linked to a number of health outcomes, including increased morbidity, mortality, changes in the immune system, and increased risk for cancers and chronic diseases later in life.
Drinking water tends to be the largest source of arsenic exposure worldwide. Based on a growing body of research, in 2001 the US Environmental Protection Agency (EPA) established a maximum contaminant level of 10 parts per billion in public drinking water. Although the EPA’s regulatory power has helped reduce arsenic exposure from public drinking water, recent results suggest that consumers may be unknowingly exposed to arsenic from a currently unregulated source, and one of the most humble of culprits.
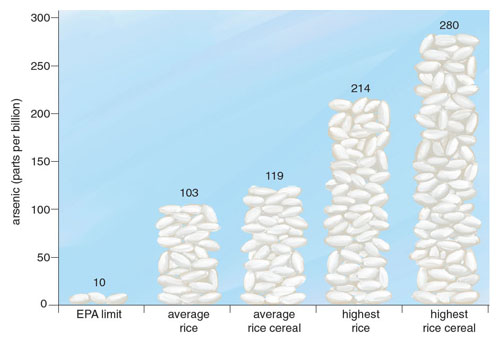
In November 2012, Consumer Reports released a report of inorganic arsenic testing performed on one of the most common food staples worldwide: rice. The results were striking; among the 223 rice and rice products tested, most exceeded the EPA’s limit on inorganic arsenic in drinking water of 10 parts per billion. Many of the samples contained arsenic levels significantly higher than this limit; the highest sampled product exceeded 270 parts per billion. Around the same time, the US Food and Drug Administration (FDA) released their own results of almost 200 rice products available for purchase in the United States and found similarly high levels of inorganic arsenic.
The national media quickly reported the findings, but many were left to wonder if this were simply another case of a mass media–fueled panic. Unfortunately, the health implications of such reports would not be so easily dismissed. Instead, they provide a new insight into a potentially significant source of chronic arsenic exposure in the United States.
The acute toxicity of arsenic has been recognized since antiquity. Known as both the “king of poisons” and the “poison of kings,” the element’s infamy grew during the Middle Ages as an almost untraceable means of murder. Nearly odorless and tasteless, arsenic could be discreetly slipped into food or drink and would all but guarantee the victim’s untimely departure, masked by the similar symptoms of food poisoning. As a result, the sudden death or illness of nobility was often accompanied by suspicion of assassination via the toxicant. For example, the Italian House of Borgia acquired considerable wealth and power through their use of arsenic-tainted wines to assassinate influential popes and cardinals during the 15th and 16th centuries.
Eventually arsenic’s use as a poison diminished once sensitive tests were developed to detect it, starting in the mid-19th century. However, its infamy is not forgotten, and modern epidemiology has shown that arsenic does not need to fall into the hands of a killer to be deadly. This metalloid—an element with metallic and nonmetallic properties—is present in every region of the globe. Arsenic is found in soil, due to its natural distribution throughout the Earth’s crust, and also as a by-product of current and historical industrial or agricultural processes such as pesticide use, smelting, and wood preservation.
Given the ubiquity of arsenic in the environment, it is interesting that reports detailing the high inorganic arsenic content of certain rice samples have been published only recently. To understand the findings, it is important to recognize arsenic’s underlying chemistry. This element may be present in either organic or inorganic forms, and inorganic arsenic is far more toxic than most organic forms found in the environment and food. In the human body, a series of successive reduction and oxidative reactions biotransforms inorganic arsenic to yield different arsenic metabolites, some of which may be more toxic than inorganic arsenic. The toxicity of each metabolite is reflective of its valence state, which determines its reactivity, half-life, and distribution in the body. As a result, each arsenic form can have different effects on the body, giving rise to large differences in toxicity.
Until recently, the most common and cost-effective method of measuring arsenic content did not differentiate between organic and inorganic forms in food, and it was thus predicted that the accumulation of arsenic in different foods was predominantly organic (arguably the less toxic form). However, advances in technologies have allowed researchers to conduct specialized testing of different products, which, in the case of rice, has revealed substantial accumulation of inorganic arsenic.
In the past 40 years, researchers have uncovered a range of health conditions in adults associated with exposure to inorganic arsenic, including diabetes, blood diseases, cardiovascular disease, and various cancers such as those of the lung, urinary bladder, skin, and kidneys. Due to the body of evidence concerning its links to cancer, the International Agency for Research on Cancer has listed inorganic arsenic as a Group 1 carcinogen, meaning it is a known carcinogen in humans. As a result of its toxicity and potential for human exposure, the US Agency for Toxic Substances and Disease Registry has ranked inorganic arsenic as the highest health hazard on their substance priority list.
Children and infants are suspected to be particularly vulnerable to the harmful effects of inorganic arsenic. In cases of in utero exposure, arsenic has been shown to readily cross the placenta. As a result, the embryo experiences exposure at levels similar to those of the pregnant mother. This is important to consider as the fetus progresses through critical windows of developmental susceptibility that can influence birth outcomes, as well as the child’s health later in life. Prenatal arsenic exposure has been linked to increased mortality, neurotoxicity, and impediment of growth, as well as changes to the peripheral and central nervous systems. In addition to health outcomes affecting the newborn, exposure to inorganic arsenic in utero has also been associated with adverse health effects during childhood and later in adulthood, such as the development of various cancers. These health endpoints are particularly worrisome in light of the recent rice testing and recent studies showing increased levels of inorganic arsenic in the cord blood of newborns whose mothers consumed contaminated rice compared to those whose mothers did not.
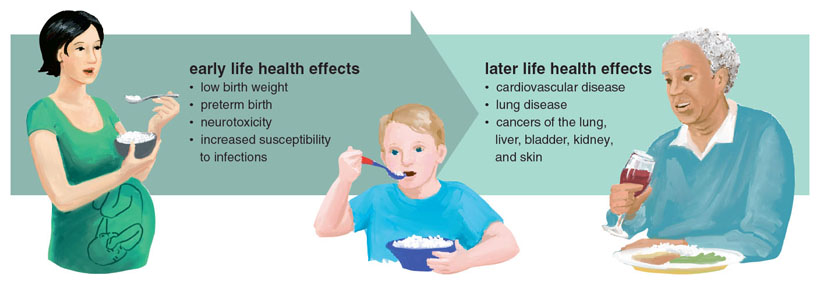
In addition to infants, children are also at increased risk due to the harmful effects of arsenic exposure. As the brain and nervous system develop, children are particularly vulnerable to the effects of environmental toxicants. A child who experiences the same or a similar level of exposure as an adult may be at increased risk because the exposure may be greater in proportion to their body weight and the enzymes needed to detoxify an agent may not be as abundant or active in a child as in an adult.
There is also the potential for increased exposure levels in children. For example, a 2009 report by the European Food and Safety Authority found that the diets of children under the age of three contained up to three times as much inorganic arsenic as the diet of an adult. In addition, researchers using data from the National Health and Nutrition Examination Study found that children who consumed rice had higher levels of total urinary arsenic than children who did not. Taken together, these exposure levels are particularly worrisome, because elevated arsenic exposure during childhood has been linked to a range of negative health outcomes in adulthood, including cardiovascular disease, lung disease, and a wide range of cancers.
Although inorganic arsenic exposure is associated with a multitude of health effects, the precise manner by which it induces toxic effects is not known. Some of the strongest experimental evidence indicates that mechanisms such as enzyme inhibition, disruption of the endocrine system, altered DNA repair, the generation of oxidative stress, and epigenetic modifications may be multifactorial contributors related to arsenic’s toxicity. Although the extent and interplay of these mechanisms are still not fully understood, it has been proposed that the metalloid may aberrantly turn “on” or “off” genes such as those that regulate critical genes and proteins that check for errors during DNA replication and repair, as well as those that control metabolism and fetal growth. Such changes in gene expression may be reflective of changes in the epigenome, a set of biological information not contained within the DNA sequence itself, but the information that influences how DNA is transcribed or translated.
The new and promising field of epigenetics is enabling researchers to study the effects of arsenic on the epigenome. Unlike some chemicals that have the potential to directly modify DNA bases, arsenic can alter the function of the genome through epigenetic mechanisms such as DNA methylation, histone modification, and changes in microRNA expression. For example, in the case of DNA methylation, many environmental toxicants can induce the addition or deletion of epigenetic “marks” or “tags” onto the DNA, which can activate or silence particular genes. Researchers studying epigenetics are beginning to unravel how and to what extent the environment, including environmental contaminants such as arsenic, can modify the epigenome. Importantly, alterations to the epigenome during critical periods of fetal development have been proposed as a plausible link between environmental toxicant exposure and health complications later in life.
Inorganic arsenic exposure has been linked to numerous epigenetic alterations, across the genome and in specific genes. Many of these genes are implicated in disease development, including those with the potential to cause or prevent cancers. These disease-associated genes have a variety of functions, such as making sure the cell can utilize nutrients, performing DNA repair, or triggering programmed cell death (a natural defense mechanism important in preventing the formation of cancer). The effects of inorganic arsenic on the epigenetic machinery is yet another possible mechanism underlying its potency as a disease-causing agent and may explain how chronic exposure to the toxicant is associated with a variety of negative health outcomes.
Arsenic accumulates in food such as rice and is therefore a potential source of children’s exposure. Rice has been described as a natural “sponge” of metallic compounds, and it can incorporate a variety of heavy metals present in soil or water, including arsenic, cadmium, and mercury. Unlike most other grains, rice plants transport silicon from the soil to fortify and protect their stalks and hulls. The same mechanisms that sequester silicon in rice can also transport and incorporate arsenic into the plant because the metalloid readily accumulates when rice is grown in arsenic-rich water or soil. The forms of arsenic that rice will absorb are affected by water and soil chemistry as well as the variety of rice being grown. As a result, certain rice species have a greater affinity than others for the accumulation of inorganic or organic forms of arsenic.
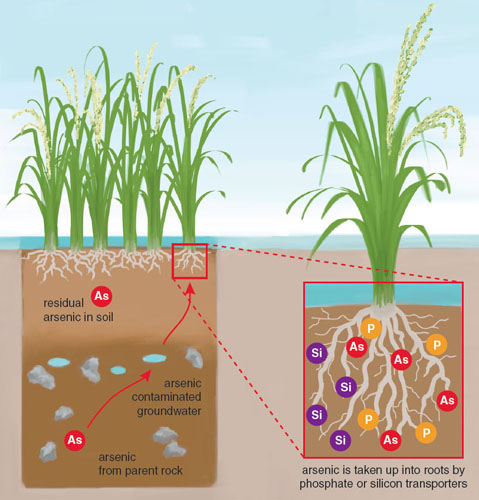
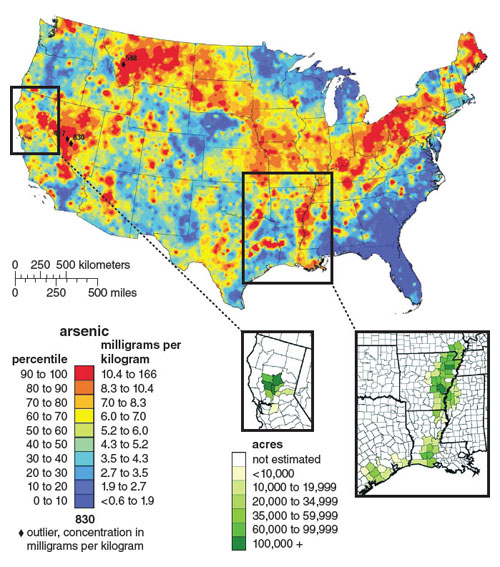
Most soil naturally contains arsenic levels of about 1 to 10 parts per billion, but in many areas where rice is grown, arsenic may be present at much higher concentrations. This may be due to natural variations in arsenic distribution, the use of arsenic-containing fertilizer or pesticide, or runoff from industrial operations. Scientists have long recognized the high arsenic content of rice grown in parts of the world with very high levels of arsenic in groundwater, such as Bangladesh. However, even in the United States, the soil on many farms used to grow rice contains high levels of arsenic, which results in the production of arsenic-rich rice. Although the soil and groundwater used to irrigate many US rice farms has substantially lower inorganic arsenic content compared to “hot spots” of arsenic contamination such as Bangladesh, the resulting rice crops can accumulate substantial levels of the toxicant that exceed the EPA’s limit for inorganic arsenic in water.
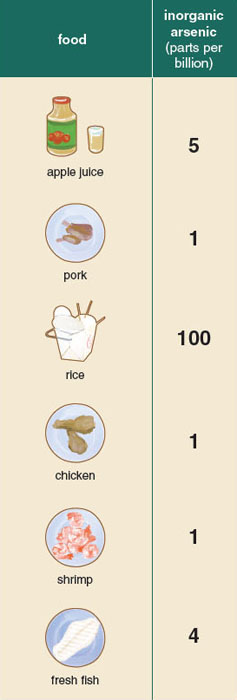
Although people may be exposed to the metalloid through the air or soil, drinking water and food tend to be the largest sources of exposure in the United States. Rice is far from the only potential source of food-based exposure. For many decades researchers were concerned about the arsenic content of shellfish. Similar to the manner in which large, fatty fish such as tuna can accumulate mercury, scientists observed that shellfish, which sift through large volumes of water as filter feeders, could accumulate high levels of arsenic. However, further testing on shellfish has revealed that although the invertebrates have high levels of total arsenic, most of the arsenic is present in relatively nontoxic organic forms.
In addition to rice, arsenic may enter our diet through a number of other crops. Many fruit orchards, such as apple and pear, are grown in soil with high levels of arsenic. Although the fruit produced by such trees can have elevated levels of arsenic, FDA testing of apple and pear juice and juice concentrates found most commercial apple juices were well below the EPA’s limit of inorganic arsenic in water. Recently, many have called for the reduction of arsenic-based poultry feed and arsenic-based antibiotics, after samples of chicken and pork were found to contain high levels of inorganic arsenic. In response, some companies actively suspended use of arsenic-based drugs in poultry and swine feed, and the FDA initiated a ban on several of these products.
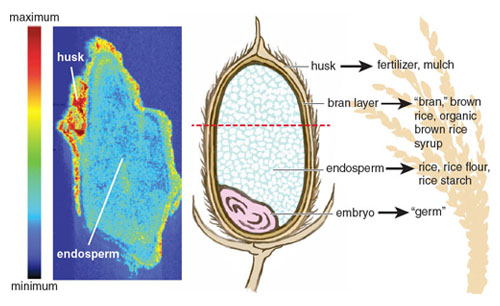
Unlike the previous examples, the arsenic content in rice and rice-based foods could be up to 100 times greater than the arsenic present in fruit, shellfish, or meat. In September 2013, the FDA released the results of a comprehensive study of more than 1,300 rice and rice-based products. As with the testing they had performed a year before on approximately 200 rice samples, the results were surprising. Of the 486 tested samples of rice available for purchase in the United States, all were above the EPA’s limit for water (10 parts per billion), with the highest level reaching almost 25 times that amount (249 parts per billion). The arsenic content of brown rice was greater than that of white rice and almost twice that of basmati rice. This discrepancy between varieties was to be expected, because arsenic disproportionately accumulates in the rice bran and husk, which are polished off in almost all commercial white rice production. Equally alarming were the results of rice-based products. The average rice-based snack far exceeds the current limit set by the World Health Organization for inorganic arsenic in water, and in many cases was higher than the average arsenic content of rice.
Individuals who frequently consume contaminated rice may be at increased risk for health effects from chronic arsenic exposure. Although the average American consumes 25 pounds of rice a year, certain populations may consume many times this amount. The results from Consumer Reports and FDA testing revealed that rice-based products, ranging from noodles to snacks such as rice cakes, contained arsenic at levels comparable to those of rice itself. As a result, individuals with dietary restrictions—such as those on gluten-free diets, those who consume rice-based products to reduce cholesterol, or individuals following diets that encourage the consumption of complex carbohydrates like brown rice—may be at additional risk.
Researchers from the Dartmouth Toxic Metals Superfund Research Program recently reported on the high arsenic content of organic brown rice syrup, a high-fructose corn syrup alternative found in many health-conscious products, including cereal bars, milk formula, and energy bars or shots. Individuals who consume products with organic brown rice syrup may also be at increased risk for arsenic-associated health effects. Even more worrisome, the researchers found many rice-based infant formulas and first foods had similarly high levels of inorganic arsenic exceeding the EPA’s limit of inorganic arsenic in water.
The FDA, tasked with protecting the safety of the nation’s food supply, is currently investigating the long-term potential health consequences related to chronic rice consumption. The agency reports that they have re-initiated an assessment for determining risk, a systematic approach to quantifying the added risk to an individual’s health from exposure to a particular agent. As part of the process, the agency will convene a panel including toxicologists, epidemiologists, and nutritionists to determine possible adverse effects following an individual’s chronic consumption of rice. This process will lead to the accumulation of quantifiable data on the range of exposures an individual may encounter, as well as health effects associated with such exposures. In cases where dose-response or health-effect data are scarce, the risk-assessment process includes standard uncertainty values used to err on the side of caution.

This same review process was recently carried out concerning the arsenic content of apple juice. Similar to the unfolding story of rice, the FDA began testing the arsenic content of commercially available apple juices after stories of the arsenic content of apple juice gained national attention. Children are perhaps the largest consumers of apple juice, and as a result, many organizations were concerned that apple juice might be a source of chronic exposure in children. Following this risk assessment, in 2013 a new action level for the arsenic content of apple juice of 10 parts per billion (the same limit the FDA set for bottled water) was introduced. Although the new action level on apple juice is meant to ensure safety, it should be noted that the arsenic levels of most of the tested apple juice products were already lower than 10 parts per billion and thus in compliance with the new rule. Rice, on the other hand, may contain arsenic levels up to 25 times higher than apple juice products. Given this proportion, as well as the lifetime frequency and ubiquity of rice consumption, it stands to reason that an action level for rice (and other inorganic arsenic–rich food) would be warranted.
At present, there exists no US regulatory limit on the amount of arsenic in any solid food product. In 2002, in an effort to reduce exposure to the toxicant and its associated burden of disease, a new drinking water standard of 10 parts per billion was enacted from the previous limit of 50 parts per billion. At this time, water was considered a greater source of exposure than food, because most food was believed to contain only small concentrations of inorganic arsenic and was thus considered to be relatively safe. The recent results of testing performed on rice by both the FDA and Consumer Reports have forced a reassessment of some of these earlier assumptions.
Certain manufactures of rice and rice products have already begun taking steps to ensure public health in light of the recent reports. For example, Nature’s One, a manufacturer of organic brown rice syrup, has developed filters to reduce the inorganic arsenic content of their product below the current EPA limit for drinking water. Certain governmental bodies have begun reviews of their own, such as the World Health Organization’s Codex Alimentarius Commission, which approved new research into the formation of limits on the levels of arsenic in food. Other countries such as China, which recently set an inorganic arsenic limit in food, already lead the United States in responding to the problem of arsenic-contaminated rice.
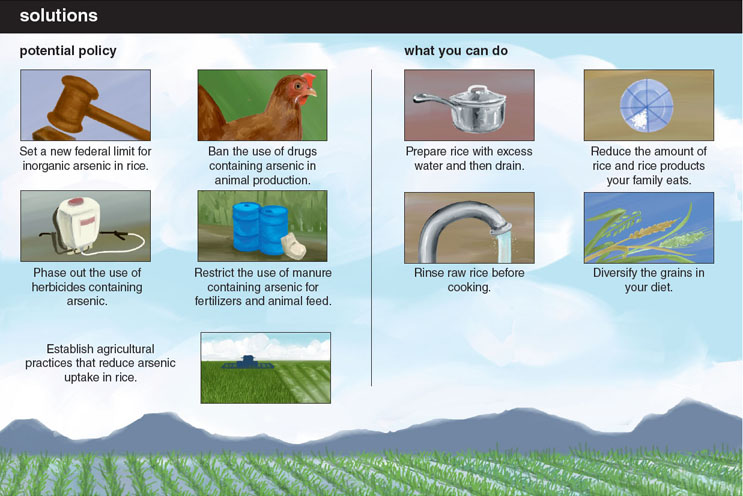
Without a definitive maximum for arsenic in rice set by the FDA, many have been left to wonder about the relative risk posed by continued consumption of rice and rice-based products. Although any concrete serving recommendations are beyond the scope of our expertise, in light of the rice-testing results, the FDA released a statement recommending “well-balanced diet for good nutrition and to minimize potential adverse consequences from consuming an excess of any one food.” A similar recommendation was put forth by the American Academy of Pediatrics to eat “a wide variety of food” to minimize arsenic exposure. The UK Food Standard Agency recently recommended that infants and young children limit the amount of rice milk and other rice-based beverages to minimize exposure to inorganic arsenic. Given the links between the health effects associated with arsenic exposure, particularly during times of developmental susceptibility, it would seem prudent to follow such recommendations.
Unfortunately, even if the FDA deems a new standard necessary to minimize dietary inorganic arsenic exposure, such limits will potentially do little to combat the global health issue. Millions around the world continue to drink arsenic-tainted water and eat arsenic-rich rice and vegetables far beyond any possible exposure faced within the United States. Closer to home, millions of US families on unregulated private wells may face similar exposures from contaminated water in addition to their rice-based arsenic exposures. For example, we have identified well water levels reaching upwards of 800 parts per billion in North Carolina. The ubiquity with which water, and now the top global food staple rice, can be contaminated with inorganic arsenic demands increased attention. Ultimately, as researchers and policy makers seek to understand and limit exposure, arsenic continues, as it has for hundreds of years, to reign as the “King of Poisons.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.