Seeing the Heart's Power
By Robert Frederick
The first 3D imaging of the intricate cardiac conduction system provides new detail for researchers and surgeons.
The first 3D imaging of the intricate cardiac conduction system provides new detail for researchers and surgeons.

Even in the fast-paced world of digital imaging, it took seven years for researchers to develop the technique to make these three-dimensional (3D) images (below). Manchester University’s Halina Dobrzynski, Liverpool John Moores University’s Jonathan Jarvis in the United Kingdom, and a team of international researchers undertook the effort because they thought high-resolution 3D imaging of an intact heart would provide new insights into how the organ works.
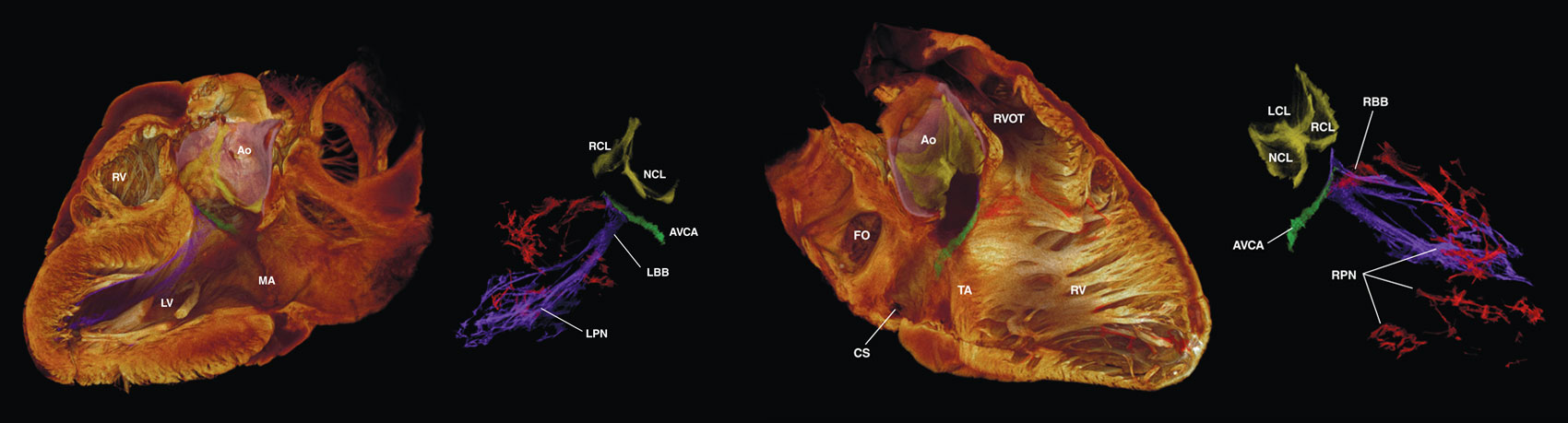
Image courtesy Robert S. Stephenson et al.
“We also pretty soon realized that in the 3D image,” says Jarvis, “we could tell the difference between the working myocardium—that’s the cardiomyocytes that produce the pumping action—and the cardiac conduction system, which is the electrical side of the heart.” The conduction system is made from specialized cardiomyocytes. Segmented conduction networks of these cells are represented by different colors and overlaid on semitransparent heart images.
Dobrzynski says the group hopes the technique will also help heart surgeons better visualize the locations of different components of the heart’s conduction system. The technique is not intended to replace any tools and imaging guides. Replacing thin-slicing and tissue-staining techniques, which have been in use for more than a century with light microscopy, is also not an option, Jarvis says. That’s because 3D imaging of an intact heart provides no interior access to the tissue to label particular proteins, which is the key to understanding what each population of heart cells is doing.
But results from the new technique already are improving upon previous research. For example, tracing the cardiac conduction system previously required researchers to follow the route layer-by-layer through perhaps hundreds of thin slices of cardiac tissue from the same heart. With the team’s 3D images has come the understanding that “commonly accepted anatomical representations [of the cardiac conduction system] are oversimplified,” the researchers write in the August 2017 issue of Scientific Reports.
Although the imaging technique is new, the technology behind it is not: Microcomputed tomography (micro-CT) scans have been in use for decades. Initially, micro-CT scanners could only image the human body’s harder tissues, such as bone and teeth, because those tissues better absorb the x-rays that give the technology its imaging power.
Imaging softer tissues has been more challenging, although many labs around the world have developed multiple techniques for doing so by injecting soft tissue with contrast agents that better absorb x-rays. That means those labs already have access to everything they need to make such 3D images of intact hearts. “I think we were successful because we knew how to handle and prepare the tissue very carefully,” says Jarvis, a muscle physiologist.
Still another challenge in this kind of research is obtaining access to normal, intact heart tissue, which is usually reserved for heart-transplant patients. With collaborators at the University of Minnesota’s Visible Heart Lab, the team gained access to human hearts that had been intended for use in transplantation, but which had not been transplanted for varying reasons. The team’s experimentation with actual human hearts was minimal, though, because they only needed to scale up from having perfected the imaging technique using the hearts of smaller mammals, including rats. “So you do have to modify your technique a little bit,” says Jarvis, such as by allowing a longer period of time for the contrast agent to diffuse into the human heart tissue. Theirs was an iodine-based agent, and that diffusion took two weeks.
That lengthy diffusion time, of course, makes this particular technique useful only with post-mortem tissue, but so too does the amount of iodine required, because iodine is toxic at such levels. Ideally, imaging techniques could guide surgeons performing real-time operations on patients, during which the cardiac conduction system is currently invisible. “It would be very unwise to say that something is impossible,” Jarvis says, “because the progress in medical imaging has been so spectacular over the last 50 years—things which we thought were impossible even 10 years ago are now possible.”
With this imaging technique, then, next steps include imaging older and damaged hearts to address whether, for example, the stretching or scarring of the heart muscle that often occurs in heart-disease patients is associated with strains on or interruptions to the cardiac conduction system.
In the meantime, the detail from these kinds of postmortem scans may be used today for exploring 3D cross-sections virtually, as shown above, or for printing 3D models, both of which can aid medical professionals in visualizing any cross-section of the heart with the visible location of the cardiac conduction system. For the 3D models, Jarvis says, “You have to use a transparent material with some colored material incorporated into the print, and that is fairly challenging, but we have done it.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.