Rationally Engineering Chemo
By Morgan Ryan
The chemotherapeutic agent cisplatin, a treatment of first resort for malignant tumors, receives a striking upgrade
The chemotherapeutic agent cisplatin, a treatment of first resort for malignant tumors, receives a striking upgrade

DOI: 10.1511/2010.87.466
The term nanotechnology evokes images of cell-sized medical bots wielding macromolecular scalpels. The real game is less picturesque, but its practitioners have a lot in common with the sci-fi image of nanomechanical tinkerers, principally the potent scientific combination of applied reason and trial and error.
In a July paper published in the Proceedings of the National Academy of Sciences of the U.S.A, Abhimanyu Paraskar of Brigham and Women’s Hospital and Harvard University, with colleagues in the United States and India, report on their effort to harness nanotech to make a better chemotherapy agent. Cisplatin is one of the top three chemotherapeutics, used as a first-line treatment for most malignancies. It works by causing cross-links to form between nucleotide bases in the DNA of cancer cells, leading to mayhem during replication and translation, rapidly followed by cell death. Unfortunately, the clinical use of cisplatin is limited by systemic toxicity, most notably to the kidney, which puts a hard limit on the safe dose of the drug. Paraskar and his colleagues have called the development of an improved cisplatin a “holy grail in cancer drug discovery.”
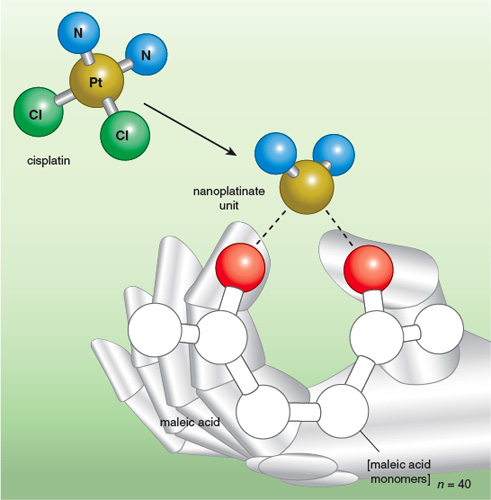
Illustration by Tom Dunne.
Increasing the viable dose of cisplatin could be a huge advance in cancer treatment. Improving its targeting of cancerous cells would help too. Nanotechnology may have opened up a route to both.
Paraskar describes the reasoning process that led to their drug-design breakthrough. First, he says, “we looked at activation. This is a small, simple molecule. We’ve known for many years how it works.” Cisplatin is activated in the cell when water molecules substitute for the two chlorine atoms attached to the molecule’s central platinum atom, in a step called aquation. The team reasoned that they should be able to devise a polymer that would take hold of platinum in place of the chlorine atoms of cisplatin. The polymer could then ferry the drug until it surrendered platinum to aquation at the right time and place—the cancer cell.
The point of attaching the drug to a polymer was to make it bigger. Large molecules accumulate preferentially in tumors. Marauding tumors need to create their own supply lines by inducing blood-vessel growth, but they aren’t very good at it. Leaky tumor vasculature and inadequate lymphatic drainage contribute to the enhanced permeation and retention (EPR) effect, one of the features of tumors that allows them to be targeted specifically with chemotoxic medications while bypassing normal tissue. It is well established that nanoparticles larger than 5 nanometers or so avoid renal clearance—particles larger than that don’t get through the storm grates of the kidney.
The nanoparticle the team chose was a string of 40 linked maleic acid monomers. Forming a one-to-one complex of platinum and polymer subunits resulted in goo. Experiments revealed that a platinum-to-polymer ratio of 15 to 1 resulted in nanoparticles of 80 to140 nanometers, optimal for perferentially homing in on tumors due to the EPR effect.
But the new particle didn’t kill cancer cells as well as cisplatin alone, suggesting that the polymer was clinging too assiduously to the platinum. So the molecule designers replaced one binding site for platinum (the thumb in the figure) with the small sugar molecule glucosamine, theorizing that the new bonding arrangement would release platinum more easily. Along the way, they learned that forming the cisplatin-polymer complex in an acidic pH favored a coordinate bond with the index finger in the figure, whereas an alkaline pH favored a coordinate bond with the thumb (a glucosamine molecule in the revised polymer). The former turned out to be the better option based on what happens in the next step in the metabolism of the medication. Once taken up in cells, the nanoparticles are directed to lysosomes, the garbage-disposal units of the cell. In the acidic pH of the lysosome, those polymer-cisplatin complexes originally formed in an acidic environment are considerably more prone to release the cisplatin they have been cradling.
Synthesis, delivery and release, all according to a rational plan. So how does the new chemotherapeutic perform? Significantly improved antitumor efficacy and significantly higher doses tolerated with no kidney toxicity—a rational-drug-design nanotechnology home run. Clinically familiar, cisplatinate in its new kidney-safe guise may take on new roles as we find out what it can do at higher doses.
Having validated the rational approach to nanotech drug design, “we are taking it to the next level,” says Paraskar. Lots of options beckon to supplant the sugar-polymer conjugate that has already proven effective at chaffeuring cisplatin to the site of a tumor. Pharmaceutical partners are monitoring developments in the tinkerer’s workshop, against the day that the concept heads into the big-money stage of human-toxicity trials.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.