Plenty of Room at the Bottom?
By William G. Eberhard, William T. Wcislo
Tiny animals maintain oversized brains, shedding light on brain evolution.
Tiny animals maintain oversized brains, shedding light on brain evolution.

DOI: 10.1511/2012.96.226
A basic fact of life is that the size of an animal’s brain depends to some extent on its body size. A long history of studies of vertebrate animals has demonstrated that the relationship between brain and body mass follows a power-law function. Smaller individuals have relatively larger brains for their body sizes.
This scaling relationship was popularized as Haller’s Rule by German evolutionary biologist Bernhard Rensch in 1948, in honor of Albrecht von Haller, who first noticed the relationship nearly 250 years ago. Little has been known, however, about relative brain size for invertebrates such as insects, spiders and nematodes, even though they are among Earth’s more diverse and abundant animal groups. But a recent wave of studies of invertebrates confirms that Haller’s Rule applies to them as well, and that it extends to much smaller body sizes than previously thought.
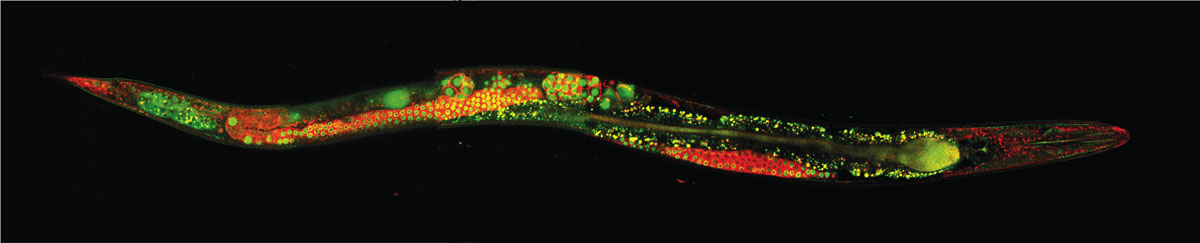
Image courtesy of Ian D. Chin-Sang of Queen’s University, Kingston, Ontario.
These tiny animals have been able to substantially shift their allometric lines—that is, the relationship between their brain size and their overall body size—from those of vertebrates and other invertebrates. Animals that follow a given allometric line belong to the same grade and changes from one grade to another are known as grade shifts. The result is that different taxonomic groups have different, variant, versions of Haller’s Rule.
The mechanisms that are responsible for grade shifts are only beginning to be understood. But this combination of generality and variability in Haller’s Rule appears to call into question some basic assumptions regarding the uniformity of how the central nervous system functions among animals. It also reveals a number of overlooked design challenges faced by tiny organisms. Because neural tissue is metabolically expensive, minute animals must pay relatively higher metabolic costs to power their proportionally larger brains, and they thus face different ecological challenges. There is reason to expect that tiny animals might cut corners wherever possible, for example by adopting lifestyles that are behaviorally less demanding. Yet available evidence indicates that at least some small-bodied animals express the same kinds of behavior as their large-bodied relatives.
Biologists have tended to ignore the lower limits of body size and the physiological processes that are associated with evolutionary decreases in brain mass. Instead they have focused on evolutionary increases in brain size, and its possible links to intelligence and other mental processes. And almost all of the current data have come from adults. But problems associated with the demands of a relatively large nervous system in a small animal are not limited to taxa with miniaturized adults. Many species have extremely small immature stages that are free-living, and whose growth and survival depends on their behavioral capabilities.
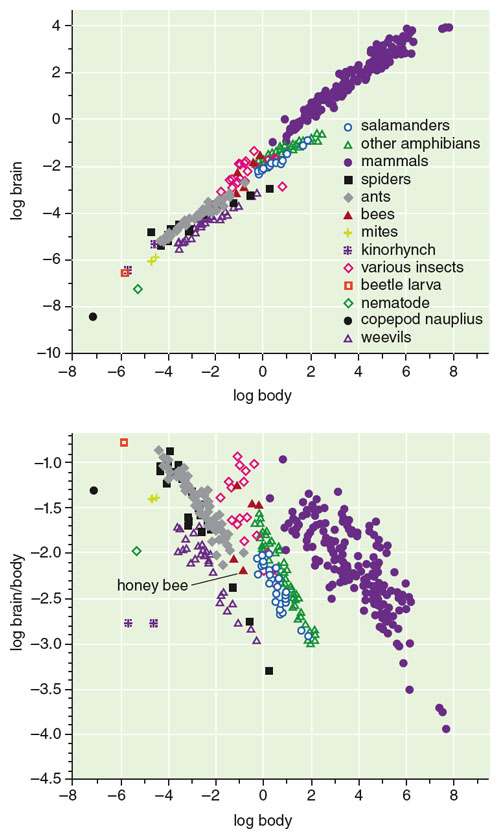
Vertebrate data are from Georg Striedter’s 2005 Principles of Brain Evolution, and invertebrate data from various sources cited in the authors’ 2011 article in Advances in Insect Physiology.
The new data on invertebrate brain allometry have several important implications. They challenge vertebrate-based hypotheses that were proposed to explain Haller’s Rule that invoked factors such as surface-volume relations, longevity and metabolic rates. They also challenge the idea, again derived from studies of vertebrates, that animals with relatively and absolutely larger brains have more sophisticated behavioral abilities and mental capabilities. For these reasons, the time is ripe for exploring the ways that central nervous systems are organized at very small scales, within constraints that differ from those of large-bodied organisms.
By focusing on evolutionary increases in brain size, biologists have generally overlooked a basic miniaturization problem that follows from Haller’s Rule: Where can a relatively large brain fit in a small body? In salamanders and fish, for example, the brain is housed within a cavity formed by skull bones. In miniaturized forms, some of these bones are lost or reduced in size, freeing up room for the relatively large brain. Arthropods have external skeletons, which they can deform to some extent to create more internal space. A nymph of the orb-weaving spider, Anapisona simoni, with a body mass of less than 0.005 milligram, appears as a speck of dust to the unaided eye. In these minute orb weavers, nearly 80 percent of the cephalothorax is filled with the brain. To house their relatively large brains, minute spiders (including tiny spiderlings of species with large-bodied adults) have a conspicuous outward bulge in the sternum, which increases the internal volume of the cephalothorax, where brain tissue is housed. In some species of spiders and mites, the relatively large brain takes up so much room that it overflows into the legs, giving new meaning to the phrase “thinking on your feet.”

Photographs courtesy of the authors.
In some groups of tiny insects, such as strepsipterans, the shape of the brain is modified to pack it tightly against internal structures and muscles. Although the “brain” is conventionally construed to be that part of the central nervous system that is housed in the head, some tiny beetles and other insects blur this distinction because they displace some or all of their large brains from the head to the thorax or even to the abdomen. In general the anatomical trade-offs that result from the displacement of other tissues have not been identified, nor have their costs been determined. The design changes imply that some features are sometimes sacrificed to house enlarged central nervous systems in very small animals, which may play a role in setting the lower limits of body size in a given taxon. A minute hooded beetle (Sericoderus; Corylophidae), for example, has fewer muscles in its head and thorax than do larger related beetles. The space taken up by the enlarged brain of a nymph of the jumping spider Phidippus clarus comes at the cost of reduced space for digestive diverticula. In both cases it is likely that there are as yet undetermined costs associated with these changes. If, for instance, it were advantageous for an adult spider to have digestive tissue in the cephalothorax, then a similar design would seem likely to have been advantageous for a nymph if it were not encumbered with a relatively large brain.
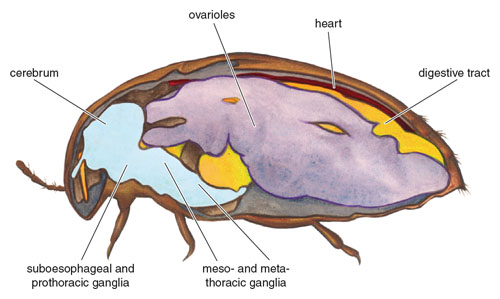
Image adapted from Alexy Polilov and Rolf Beutel’s 2010 article in Arthropod Structure and Development.
Another approach to solving the housing problem would be to reduce neuron size, thus reducing overall brain size while maintaining similar numbers of neurons and the degree of connectivity. The sparse available data suggest, however, that such adjustments are incomplete, and that within any given taxon, smaller animals usually have fewer neurons. Reductions in neuron size are possible, but only to a point. Nobel laureate physicist Richard Feynman discussed general limitations regarding storing and retrieving information at extremely small scales and concluded that “there’s plenty of room at the bottom” when building artificial information-processing systems at nanoscales. In biology, however, neuron-based information-processing systems bottom out at sizes where Feynman just gets going. There is a theoretical lower physical limit on the functional diameter of an axon (about 0.1 micron). Below this size, an axon can no longer transmit reliable information because the signal is swamped by noise from spontaneous depolarizations of the membranes. In addition, the minimum size of a neuron cell body is limited by the size of its nucleus, which in turn is limited by the animal’s genome size. The nucleus comprises up to 80 to 90 percent of the volumes of small neuron cell bodies in tiny insects. One route to miniaturization among arthropods would be to delete chromosomes, pack the chromatin more tightly or eliminate the nucleus, which would permit a smaller neuron. Such modifications had been documented in vertebrates but were unknown in invertebrates until just recently. Alexy Polilov of Lomonosov Moscow State University has shown that most of the neurons of minute parasitic wasps, Megaphragma sp. (Trichogrammatidae), with body lengths of 170 to 200 micrometers, lack nuclei. The pupal central nervous system has about 7,400 nuclei, but near the end of pupal development most neuronal cell bodies break open, or lyse, and lose their nuclei. The adult central nervous system, therefore, has about 7,000 cells without nuclei, and only 339 to 372 cells with nuclei, of which only 179 to 253 are in the brain.
Lysis also is associated with volumetric changes in the nervous system. The pupal brain volume of about 93,600 cubic micrometers decreases to 52,200 cubic micrometers in the adult. In addition, numerous folds are present in the cuticle of the back of the head, the occipital area, and the size of the head capsule in this area is reduced. Remarkably, the central nervous system of M. mymaripenne has orders of magnitude fewer neurons in comparison with other flying insects, such as Musca flies, which have 340,000 neurons. A somewhat larger trichogrammatid wasp, Trichogramma evanescens, for example, has 37,000 neurons in just one part of the brain, the supraesophageal ganglia. Despite their extreme central nervous system modifications, Megaphragma wasps nevertheless perform behavior such as mating, flying, host searching and recognition, although the details have not been studied. The kinds of compensatory mechanisms that enable this behavior with only a greatly reduced number of neurons, most of which lack nuclei, are not known.
Energetically, the brain is a gas-guzzler, as neural tissue is more expensive to run than most other tissues. Humans, for example, have brains that account for slightly more than 2 percent of our biomass, but they burn up more than 15 percent of our basal metabolic energy. In addition, the density of information processing is greater in a smaller brain when it is performing the same operations as a larger brain and is thus more costly on a unit basis. These differences imply that the strength of natural selection for energetic efficiency may be more intense in small than in large species, especially within a given grade. They also raise the question of how tiny animals pay these high energetic costs.
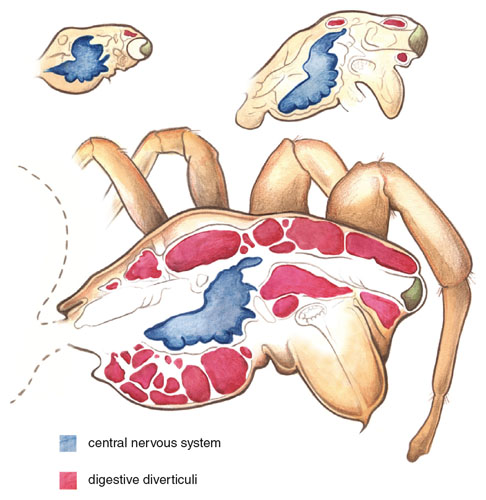
Image adapted from David E. Hill’s 1975 master’s thesis at Oregon State University.
With respect to information processing, animals might adopt several non-exclusive strategies to reduce costs. One, the size limitation option, is to reduce behavioral capacities and thus reduce the amount of neural tissue needed to sustain behavior. Another is the oversized brain option, which involves maintaining behavioral capacities and bearing the high metabolic costs of an enlarged central nervous system. And then there is the economy-of-design option, or modifying the properties of neurons and neural networks to increase behavioral capability per unit of nervous tissue. The last option could allow smaller animals to produce comparable behavior without investing in large nervous systems.
There are several ways that animals might achieve economy of design and thus economize on the energy budgets of their brains. All animals have sensory receptors that are tuned to specific inputs to improve their efficiency. Matched filters take advantage of mechanical properties of sensors and stimuli to filter sensory input without expending energy to do so, which reduces processing expenses at the receptor and at higher central nervous system levels. Many insects, for example, are highly sensitive to polarized light, a trait associated with the physical alignment of rhodopsin molecules in the microvillar membranes of photoreceptors. There are numerous other possible opportunities to reduce central nervous system costs, such as a heavier reliance on analog transmission and graded depolarizations, which work at small distances and are energetically more efficient than the action potentials that many other animals use for communication among neurons. Another example includes the use of the same neurons for multiple tasks, such as both sensory and motor functions, as is common in nematodes. Neuromodulation may be used more frequently by small animals. That involves altering the effects of a neuronal circuit by exposing it to different chemical environments, producing different behaviors. Nematodes frequently use muscle plates that allow a single synaptic process to stimulate multiple muscles. Still other strategies include the indirect control of cilia through muscles; a reduction in the relative numbers of interneurons, which transmit signals from one neuron to another as opposed to sensory and motor neurons; and the rearrangement of neuron positions and connections in order to minimize the total length of axons and dendrites. That last design is analogous to an architect holding down building costs by minimizing the length of wire needed to provide electricity throughout a house. The nervous system of the nematode Caenorhabditis elegans has been analyzed with respect to an optimal design in this save-wire aspect. The trend toward greater fusion of different ganglia in the central nervous system of minute insects may also be related to this type of efficiency. The relative frequencies of such design features in large versus small animals are not known.
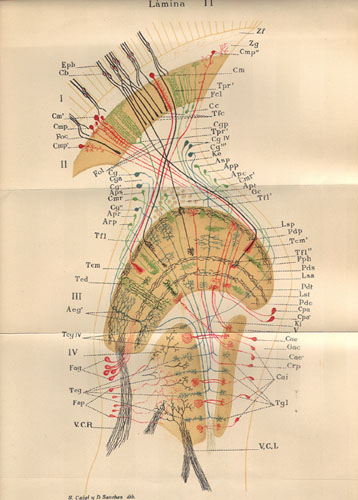
Image courtesy of Trabajos del Laboratorio de Investigaciones Biológicos de la Universidad de Madrid.
Other possibly important differences in small nervous systems involve the neurons themselves. In at least some groups, the dendrites of smaller neurons are themselves smaller and said to be less complex. Nearly 100 years ago, insect neuroanatomist Santiago Ramón y Cajal produced pioneering—and beautiful—studies of the nervous systems of insects. He found insect neurons to be more elaborate than those of vertebrates, and likened the neuroanatomy of an insect to a “fine pocket watch,” as opposed to the “rough grandfather clock” neuroanatomy of a vertebrate. In general, the functional significance of these neuroanatomical differences is not well understood, but we expect that they represent promising starting points for future research.
Design economies could also occur at the behavioral level but are even less studied. Robotic engineers incorporate behavioral design features to minimize the input needed to generate information about the external world in order to achieve desired outcomes. A rigid hierarchy of relatively simple behavioral subroutines can facilitate goal-directed behavior if subroutines are serially ordered to maximize desired motor output per bit of sensory input. For instance, grasping a randomly positioned object is a difficult task that requires a series of visual-to-motor transformations. In robots this task is rendered easier computationally if a robot executes a subroutine to turn and face the object, triggering a second subroutine to move to a specified distance from it. Grasping it from a standard distance and direction simplifies the task. The possibility that small animals are more likely to use such behavioral designs has, to our knowledge, never been checked.
Grade changes reflect increases or decreases in the slopes and elevations of brain-body scaling relationships in different taxa. Evolutionary transitions between grades are poorly understood, but are obviously crucial to understanding how nervous systems function and how the diverse array of animal body sizes has evolved. Most previous discussions of grade changes have emphasized evolutionary transitions toward larger body size and more highly encephalized forms, probably due to a general fascination with the possible implications for greater intelligence. The new invertebrate data highlight evolutionary changes in the opposite direction—miniaturization—and the attendant central nervous system design problems at minute body sizes. A grade change can make it possible for much smaller body sizes to evolve than would have been possible if the animals had continued to use the slopes and elevations of brain-body lines from the previous grades. For example, if a 1-milligram animal used the scaling rule used by salamanders, it would have a brain that constituted a prohibitive 20 percent of its body, a proportion that is larger than that of any known animal. Grade shifts suggest that some particular taxa of small-sized animals, such as ants, have apparently solved scaling problems that seemed insuperable for animals of larger-sized taxa, although they do not explain how or why.
The changes in design that are associated with most grade shifts remain to be worked out, but it is likely that they are at least sometimes associated with the evolution of new neural design mechanisms. This can be illustrated by comparing two groups that are extremely different—the neuron-miserly nematodes, and the neuron-profligate vertebrates. The nematode C. elegans has a nervous system with only 302 neurons, and some other nematodes and tiny invertebrates have even lower numbers. Each C. elegans neuron has only about 25 synapses, and the neurons are connected in highly consistent and relatively simple ways.
The contrast between the brain of a nematode and that of a human could hardly be greater. Our brains have astronomical numbers of neurons, an estimated 85,000,000,000; huge numbers of synapses per neuron, such as 10,000 per pyramidal cell in the cortex; and extremely high degrees of connectivity. For example, there are roughly 101,000,000 possible circuits in the human cortex alone, which prompted Nobel laureate Gerald Edelman to describe the human brain as “the jungle in the head.” The functioning of our brain depends on populations of neurons—on the activity patterns in recurrently interconnected populations of neurons—rather than on the activity of individual cells. The activity of individual neurons is neither consistent nor useful in processing information, and patterned activity of neuronal populations can emerge in alternative ways from different subsets of the population. A single dysfunctional neuron can thus be inconsequential for a vertebrate but catastrophic for a nematode. The loss of one particular neuron in C. elegans, for instance, brings evolutionary fitness to zero because it leaves females unable to lay eggs.
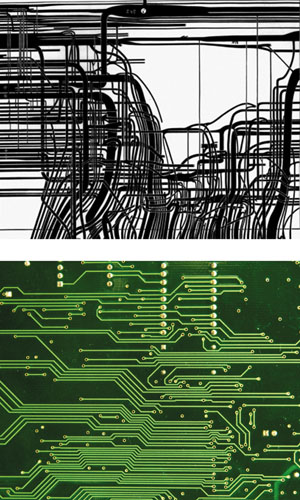
Diagram at the top from Advances in Insect Physiology, Elsevier; photo from iStockphoto.
The striking visual resemblance between the circuits on a computer chip and a wiring diagram of nerve fibers in the ventral nerve cord of a nematode draws attention to the computer-like traits of a nematode’s nervous system, with its fixed number of neurons with invariant connections. This design stands in contrast to the decidedly noncomputer-like traits of vertebrate nervous systems. The processes by which these two extreme types of nervous systems are built up as an organism grows are also strikingly different. Growth of the vertebrate nervous system is characterized by an extraordinary overproduction of neurons in young stages, followed by selective pruning of inactive neurons and synapses. The extent to which neurons die during development varies among, and within, species. For example, the percentage of retinal ganglion cells that die during development varies from 80 percent in cats to 60 to 70 percent in rats, mice, rhesus monkeys and humans, and to approximately 40 percent in chickens and amphibians. Selective pruning of neurons in nematodes is, on the other hand, nearly nonexistent. In C. elegans a total of 8 of 310 neurons, 2.6 percent, are discarded as the animal matures, and they are always exactly the same cells, resulting in the 302 neurons of the adult hermaphrodite; there is no indication that use or disuse is a factor influencing cell death. Similarly, a second instar spiderling and an adult of the orb weaver Argiope aurantia have approximately the same number of neuronal cells, despite an approximately 24-fold difference in total brain volume. Such extreme contrasts raise the possibility that nervous system function differs profoundly in different parts of the animal kingdom, in contrast to the generality of much of biochemistry, molecular genetics and molecular development. The miserly design typified by nematodes may represent adaptations that permitted the evolution of miniature body sizes.
The common supposition derived from vertebrates—that animals in lower grades are necessarily inferior or less capable in their behavior—is not well supported by facts. Among adult orb-weaving spiders that vary in body mass by 400,000 times, including those near the lower size limit for spiders, there is no evidence of inferior performance with respect to the precision of executing certain web construction behaviors or the ability to adjust web designs to local conditions or to build alternative web designs. Qualitative observations also suggest that size limitation of behavior fails to explain some other grade differences, such as that between honey bees and salamanders. Miniature salamanders and honey bees have approximately the same body size, but the latter fall on an allometric line lower than that of the salamander. A honey bee is nevertheless capable of such feats as navigating using landmarks and polarized sunlight that is adjusted for time of day; learning complex patterns involving different sensory modalities or general concepts such as different or similar and above or below; and using language to tell nest mates where to find food. It would be difficult to defend the argument that a honey bee is behaviorally inferior to a miniature salamander.

Kim Taylor/Warren Photographic Ltd.
Even the neuroanatomically simple nematodes behave in ways that are not fundamentally different from many animals with many more neurons. For example, C. elegans senses and responds to various stimuli, including physical contact with environmental objects, and perceives various chemicals, oxygen concentration, osmolarity, pH, temperature, light and pheromones. These various inputs are used to coordinate motor output, and to evaluate conditions such as the density and sex of conspecifics. Motor outputs include different movements for swimming and for crawling on a surface, for turning or reversing those movements or for altering them after fixed time periods. These animals orient and move toward or away from point stimuli; forage for food, engulf it, perform rhythmic swallowing movements and defecate; seek mates, copulate and lay eggs. In addition, nematodes can learn to modify a variety of motor behaviors on the basis of their experience.
The idea that grade changes are associated with differences in behavioral capabilities in different species stems from an assumed correlation between brain size and behavioral capabilities. Comparative studies have often been confounded by the use of imprecise, difficult-to-define behavioral metrics such as intelligence. And an important question remains unanswered: How do we quantify and compare behavior in biologically meaningful ways across different taxa? The size of an animal’s behavioral repertoire has sometimes been used as a quantitative measure of a species’ behavioral complexity. This intuitively appealing idea suffers from several problems. In a pioneering study relating behavior to head and brain size in ants, Blaine Cole, now at the University of Houston, highlighted some of them. Such a metric relies on subjective decisions to distinguish specific behaviors, and faces a problem common in artificial classification systems. Observers who are “splitters” will recognize a larger number of behaviors than those who are “lumpers.” In addition, repertoire comparisons rely on a number of unverified assumptions: that each behavior is equally demanding in terms of neural system processing, that behaviors called the same thing in different species are equally demanding and that rare behaviors, which are more likely to be missed by observers, are not drivers of brain evolution. It’s also assumed that the speed and precision with which a given behavior is performed is the same in different species and that the environmental influences shaping behavioral expression are minimal. It also makes the unlikely supposition that laboratory colonies will reveal the full repertoire of important behaviors.
These problems have led some researchers to adopt other metrics, such as the frequency of mistakes when making decisions, or the degree of precision in adjusting behavior to other variables. These traits can be compared more consistently across diverse species. Behavioral precision, the ability to accurately and consistently reproduce the same behavior, has been hypothesized to be less developed in relatively small-brained animals. Mistakes or imprecision might arise in smaller animals in several ways. For one, they have fewer sensory receptors, and thus should have decreased sensory input and hence less reliable information about the environment. They have less thorough processing of sensory inputs because of fewer interneurons or dendrites. Motor output or coordination among different limbs may be compromised by reduced feedback from proprioceptors, which sense stimuli from within a body, or from increased noise in the nervous system. The available data from tiny orb weaving spiders do not suggest behavioral limitations and are not consistent with the size-limitation hypothesis. Tiny spiders are morphologically modified to house a relatively enlarged brain, suggesting that they have adopted strategies consistent with the over-sized brain alternative we have described. The generality of this finding is currently unknown, however, because of the lack of comparative data. Other behaviors have not yet been thoroughly explored in spiders and other tiny animals. One that might be meaningfully compared among different species is the ability to learn and remember different types of lessons.
Finally, the use of overly inclusive measurements, such as overall brain size, rather than measurements of regions involved directly in behavioral tasks, can confound comparisons among species. Bats, for example, rely on hearing to a greater extent than we do. Due to body size differences, our subcortical auditory brain region is vastly larger than that of bats, so overall size is not informative. Relative size, however, reveals that the subcortical auditory region of the bat brain comprises 1.6 percent of total brain volume, versus 0.015 percent in humans. The problem of associating behavior with particular brain regions is exacerbated by cultural differences among scientific disciplines. Studies on behavior often take a comparative approach and include data from diverse species. Neurobiological studies are more often focused on a very small number of so-called model organisms that are studied under laboratory conditions, where they express a much smaller range of behaviors than in nature. Indeed, one recent study showed that approximately 75 percent of the research efforts of neuroscientists, as judged by numbers of publications, were directed at only three species—the mouse, the rats, and the human. When compared with a recent estimate of 7.7 million animal species worldwide, that is about 3.9 x 10-5 percent of animal biodiversity.
Problems associated with miniaturization are much more general than they might first appear. In addition to the many species with miniaturized adults, there are many more species with moderate-sized adults that have very small, free-living, immature stages. A mature female of the giant golden-orb-weaver Nephila clavipes weighs on the order of 2,000 milligrams, for example, but each of her newly emerged spiderlings weighs only 0.7 milligram and has tell-tale adjustments to small size, including extensions of its brain into a bulging sternum and into its legs. The ecological problems relating to energy intake and expenditure of smaller, young individuals are likely to be different from those of larger conspecific adults. Because of their higher metabolic costs, smaller animals may be more likely to be living on the edge of energetic limitations and less buffered against temporary food shortages. An increased susceptibility to unfavorable energy balances could have significant biological repercussions, such as limiting an animal’s geographic distribution or its ability to survive food shortages or other stresses, and might select for alternative ecological strategies. Parasitic wasps (Hymenoptera) that use insect eggs as hosts are among the smallest known adult insects, perhaps because their larvae hatch into a host environment that contains all the necessary nutrients, thus permitting greatly reduced egg sizes and lower energy reserves.
Small immature stages of species may represent the leading edge of evolutionary innovations to solve size-related physiological problems as a lineage evolves toward smaller body size. Are grade changes associated with innovations that result in energetic efficiencies in the central nervous system? For instance, weevils have a low allometric brain-body line compared with many other insects. Are there economies of design that make their nervous systems more efficient in generating behavioral abilities? And are these efficiencies associated with the evolutionary and ecological success of weevils, one of the most speciose taxa of all animals? Answers to such questions are unknown, in part because they are rarely asked. We agree with Princeton biologist John Bonner, who emphasizes in a recent book that “size matters” in ecology and evolution. An understanding of the evolution of brain form and function requires comprehensive data on neuroanatomy, neurophysiology, behavior and ecology from a diverse array of species, not just a few models. Studies dealing with the nervous systems and behavior of very small animals are likely to reveal phenomena not seen in the larger animals that are typically studied. Tremendous opportunities await for neuroethological studies of taxa with miniature animals, and syntheses of data and ideas from disparate fields such as brain allometry, animal behavior, ecology, neurobiology, classic invertebrate zoology and molecular and developmental biology. An understanding of the patterns and processes involved in evolutionary decreases in body and brain size is also likely to illuminate those associated with evolutionary size expansion, and both are likely to further our understanding of crucial evolutionary transitions from one evolutionary grade to another.
We can’t conclude this discussion of brain and body sizes without acknowledging that we have ignored the proverbial 500-pound gorilla lurking in the room throughout this discussion. We have examined many different consequences of Haller’s Rule, but we have given no explanation for why the rule should be true. Why should organisms ranging from the different castes of ants in a single nest, from primates to salamanders to beetles, so consistently have relatively larger brains when they have smaller bodies? The new invertebrate data help by ruling out some previous explanations that were only reasonable for particular groups of vertebrates. But we do not have an alternative general explanation. It is very unusual in biology that such a general trend as Haller’s Rule should have such a depauperate array of hypotheses lined up as possible explanations. More often the problem is having too many competing ideas to test. Understanding why Haller’s Rule is so generally true is likely to be important in answering central questions regarding the evolution of the central nervous system and how and why different grade changes evolved.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.