Performing Power
By Lee Alan Dugatkin
In conflicts between animals, factors such as who won previous bouts and who is watching may play a role in the outcome.
In conflicts between animals, factors such as who won previous bouts and who is watching may play a role in the outcome.

Anyone who has watched ravens (Corvus corax) being ravens needs no convincing about how sociable and intelligent they are.
Thomas Bugnyar, an animal behaviorist now at the University of Vienna, had certainly seen his share of these remarkable birds, but he never expected he’d spend the better part of his life studying just how sociable and intelligent they are, nor how those characteristics play into raven power structure. Then one day he visited a friend at the Konrad Lorenz Field Station in the Northern Austrian Alps, about 230 kilometers east of Vienna. The friend was part of a team hand-raising ravens at the field station. Bugnyar recalls, “I was quite impressed by the birds, because they were not actually behaving like birds, but more like dogs and puppies.”
Not long after that visit, Bugnyar joined the raven research team himself, and one of the birds made him a true believer in all things raven. The ravens he was working with were housed in pairs, and a raven in one pair had escaped. The other one “was all by himself for a few months,” Bugnyar says, “and during that time I spent every lunch break with him, playing with him, just to give him a little contact, and I also encouraged everyone else to interact with him, because he was bored.”
Bugnyar would occasionally give his raven friend a piece of cheese as a snack, and one day he had a small slice in his pocket and reached in to get it as the bird sat on his arm. “I showed him the piece of cheese,” he continues, “and as soon as he saw it, he went for it in a very fast way, and so I pulled back my hand, because he came so fast. The beak is big and if it does not hit the cheese, but your finger, it hurts.” The raven looked him squarely in the eyes and said “aua!” which happens to be the German equivalent of “ouch!”—an exclamation the raven had no doubt heard used by one of the many humans who had befriended him. “I said, ‘Wait a second, no, almost aua, you behaved yourself,’” says Bugnyar. “My interpretation was that he anticipated my expression [‘aua’] in response to his behavior. He was using [a human] expression in the perfectly right context. His social environment at that time was humans, and so he was trying to make sense of what we were doing. It also illustrates quite nicely why I ended up using vocalization as a window into what they actually think.” And, Bugnyar would come to learn, into their power structure as well.

Nature Picture Library / Alamy Stock Photo
Year in and year out since his graduate school days, Bugnyar has returned to the station to continue his now 25-year-long study. Armed with binoculars and tape recorders, he and his students have seen—and, just as importantly, heard—thousands of power-related interactions. Struggles for power take many forms in ravens, including approach–retreat sequences, in which one bird backs off as soon as another approaches; forced retreats, during which a raven retreats after being threatened; and true fights, when birds grapple using their very sharp beak and claws.
From the birds’ perspective, Bugnyar and his team of observers are not worth paying any mind to. But audiences made up of other ravens are a different matter. Ravens who are victims of aggression are known to give defensive calls, and Bugnyar and his team have found that bystanders sometimes come to the aid of these victims. But it seemed to him that there was more going on than a simple cry for help. “Sometimes when there is a beat-up, [the victim] seems to cry like mad . . . even if it is a mild beat-up . . . they seemed to me to overdo it,” he says. “At other times, there is a pretty intense beat-up and they seem to stay quiet.” He began thinking that the composition of the audience of ravens who were watching and listening might be what made the difference. In 2010, Bugnyar and his graduate students Georgine Szipl and Eva Ringler decided to dig deeper.
Ravens on the wrong side of a power struggle modulated their defensive calls depending on the nature of the audience: Their call rates were higher when potential allies were audience members.
The team videotaped forced retreat interactions in which the victim uttered a defensive call and noted the duration and number of those calls. They also collected information on the identities of other ravens within 25 meters of the interaction. Using their long-term database, they then classified each bystander as kin (or not) to the victim or aggressor. They also scoured their records to see whether a bystander had a strong social bond with the victim or the aggressor, as measured by whether the two were mating partners or had exchanged affiliative behaviors such as grooming.
Bugnyar and his colleagues found that ravens on the wrong side of a power struggle modulated their defensive calls depending on the nature of the audience. Victims’ call rates were higher when potential allies—either kin or those with whom the victim had a strong bond—were audience members. When the audience was made up of individuals who had a history of prosocial behaviors with the aggressor, victims, who might suffer by drawing even more attention to their predicament, reduced their call rates.
Ethologists have come to learn that paying attention to who is in the audience is just one of the many tools that animals use to help them acquire power. Gathering intel in all its forms is favored by natural selection.
Klaus Zuberbühler of the University of St. Andrews studies the behavior and neuroscience of nonhuman primate communication. When in the early 2000s he began studying chimpanzees (Pan troglodytes) at the Budongo Conservation Field Station in Uganda, one thing became obvious very quickly: Chimpanzee power struggles are loud. Both aggressors and victims scream, and Zuberbühler observed that “depending on who is listening, they tend to exaggerate, more or less, about the nature of the attack.” Just like the ravens. He began to think that the nature of the audience was the key to these vocalizations. “If you are being attacked . . . [often] the only way to get out of it is to get someone else to join and that may turn the tide. If the [victim’s] scream recruits help, then it really matters who is nearby. Especially if it is the alpha male, who does not tolerate violence among others.”

Karl Ammann / Nature Picture Library
Zuberbühler teamed up with Kate Slocombe of the University of York in England to analyze 84 chimp power struggles. They found that when fights involved only mildly aggressive interactions, the animals took no account of whether an audience was present. When contests involved severe aggression, victims’ screams were longer and more intense when an audience was nearby—but only when at least one of the audience members held a rank in the dominance hierarchy that was equal to or above the rank of the aggressor. This strategy seems to pay off: Victims that emitted longer and more intense screams received support from high-ranking observers who often intervened and broke up fights.
Chimps and ravens are hardly alone when it comes to the role an audience plays in power dynamics. Audience effects have been found in Japanese quail (Coturnix japonica), fiddler crabs (Uca maracoani), zebrafish (Danio rerio), and Siamese fighting fish (Betta splendens). In fighting fish, it gets particularly interesting. A male’s testosterone level changes when he is being watched, and some work suggests that fighting males change their behavioral repertoire if a female, but not another male, is watching, and that all of these changes are mediated by whether a male knows those in the audience.
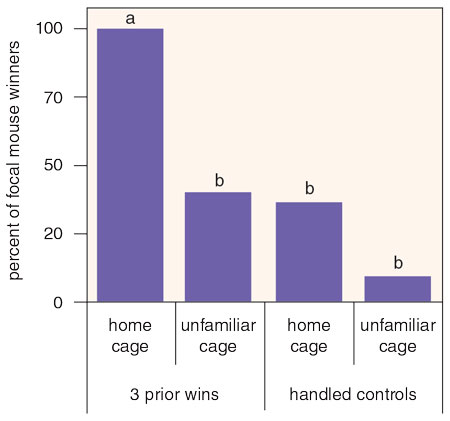
Audience effects are just one of a class of power-related phenomena called extrinsic effects. In contrast to intrinsic effects, such as size and weight, extrinsic effects incorporate various aspects of the experience and the social environment of those seeking power. In addition to audience and bystander effects, two other types of extrinsic effects are winner effects and loser effects. Winner effects occur when an animal’s chance of winning a contest for power increases as a function of prior wins. When the probability of losing increases as a function of prior losses, loser effects are in play. Of the two, loser effects seem to be more common, and ethologists such as Gordon Schuett, codirector of the Chiricahua Desert Museum in New Mexico, have turned to copperhead snakes to better understand just why that is.
Most teenagers want to turn their basements into game rooms, not laboratories housing dozens of venomous snakes that they’ve caught. But most teenagers are not budding herpetologists like Schuett was back in the day. “I was very fascinated with venomous snakes as a young boy,” Schuett says. “I was collecting rattlesnakes when I was 15.” Soon, he was not only milking the rattlesnakes for their venom, but as a high school student was reading the primary scientific literature on all things venomous snake.
At age 16, Schuett became obsessed with power dynamics in copperhead snakes (Agkistrodon contortrix). “I was utterly struck by male–male combat [and thought,] ‘I’m going to try and do that in my basement.’ . . . And lo and behold, I was able to get them into combat.” Four decades later, he’s still enthralled by power struggles in venomous snakes.
Copperheads are solitary creatures, except in the late summer and then again in the spring, when they gather in large mating aggregations. When they fight, copperheads live up to their species name, which translates from Latin roughly as “twisted fishhook.” During contests, males contort themselves in every conceivable way, angling for leverage. Power contests typically begin with challenge displays, including ascend, in which a snake rises up from the ground, and sway, in which it undulates back and forth. If the challenge displays don’t settle the contest, a male sometimes tries to hook his opponent, positioning himself above and around the other snake. If he is successful, the opponent is often forced to the ground. If snakes are simultaneously hooking each other, they intertwine and stiffen, eventually separating, with one male emerging as the victor and the other showing submissive behavior and retreating.
Initially, Schuett watched these power struggles from behind a blind in the lab, but he soon realized that was unnecessary: “They didn’t care if I was there,” he says, laughing. “When they wanted to fight or court, they did.” As he watched, it struck him that losers not only retreated, but went into what he called a refractory period, during which they avoided aggressive interactions with anyone. Schuett had read about such behaviors in other species—mostly fish—when he was doing this work in the early 1980s, but in those studies the effect lasted hours, whereas for his snakes the refractory period seemed to last a week or more.
Schuett ran a series of experiments in which two copperhead males were paired near a female during the late summer mating season. In the first of these experiments, neither male had had a recent losing experience, but one male was about 10 percent larger than the other. Thirty-two such triads were formed, and the larger male won every single time, subsequently courting and guarding the female. The next day, Schuett took 10 of the winners and 10 of the losers and pitted each one against a similar-sized male who had no prior fighting experience. What he discovered was that copperheads don’t go on winning streaks: First-round winners were no more likely to win than were their opponents. But losing is very bad news. Males who lost in the first round were never the first to challenge their next opponent, and in each and every fight, they retreated. The winner then courted and guarded the female. When Schuett ran the same sort of experiment seven days after a loser had lost, he found very similar results. Losing begat more losing.
Schuett wondered what would happen if he paired a snake that had recently lost against an opponent that was 10 percent smaller. Would the size advantage compensate for the prior loss? The answer was a resounding no: The losers lost again. Lose once in the battle for copperhead power, and the effects linger and create a serious fitness cost.
But why would it be adaptive to effectively shut down the quest for power for a week when the whole mating season lasts for only a month? One possibility is that the life span of a copperhead is long enough that things might get better at some point down the road, so it pays to temporarily shut down. “If you engage in combat and become the loser,” says Schuett, “in theory you have lost one-quarter of the mating season. If you lose again, you may never want to engage in courtship again that season. I think they can only manage that burden because these animals can live 25 or 30 years.”
Schuett next asked what was causing loser effects in real time. That is, at the physiological level, what was making losers more likely to lose again? To find out, he and his colleagues again put pairs of males together near a female and waited until one male was the clear winner. At that point, they separated the males and took a blood sample from each. For comparison, they also ran two controls: In the first, they took a blood sample from a lone male; and in the second, they placed a single male and a female in the arena, then took a blood sample from the male. Analysis of the samples revealed that plasma corticosterone, a key stress hormone, was significantly higher in losers than in winners or control males, suggesting that increases in stress hormones might signal to loser males that they should shut down the quest for power and wait for better times.
Cathy Marler knew of the work on loser effects in copperheads. Indeed, she could find many examples of loser effects in the animal behavior literature. But what of winner effects? Marler, a professor of psychology at the University of Wisconsin, occasionally came across such effects in her work, but any boost from winning seemed short-lived. Although it was true that some mathematical models suggested that loser effects were more likely to evolve than winner effects, Marler felt something was missing. It just didn’t make sense to her that winner effects should be transient when they seemed so important in helping animals gauge the social environment and determine when to ramp up aggression.
Marler’s work focused on the behavior of two mouse species: the California mouse (Peromyscus californicus) and the white-footed mouse (Peromyscus leucopus). It is hard to imagine two species that are so closely related but have such different social systems. Both look like your standard mouse, but California mice are monogamous, whereas white-footed mice are polygamous, and male and female California mice provide more parental care than their white-footed counterparts. California males are also more aggressive to intruders than are white-footed males, which may in part be due to their having more brain receptor sites for a hormone called arginine-vasopressin (AVP), which is known to promote aggression in male, but not female, mammals.
From M. J. Fuxjager, et al. Hormones and Behavior 56.
To dig deeper into AVP’s role in shaping aggression and the path to power in these two species, Marler conducted a cross-fostering study. Cross-fostering experiments with two species involves raising the offspring of individuals of Species A in the nest of Species B, and vice versa. If offspring behave like their foster parents when they mature, it suggests that the developmental milieu strongly affects behavior.
Together with Janet Bester-Meredith (now an associate professor of biology at Seattle Pacific University), Marler raised 24 white-footed pups with California foster parents, and 14 California pups with white-footed foster parents. When foster offspring were about seven months old, they were tested in standard aggression tests that had been developed for rodents. The mice generally behaved more like their foster parents than their biological parents. The strongest effects were found in California males, who showed much lower levels of aggression when they grew up in the nest of white-footed foster parents. This change was partly due to the effect of cross-fostering on AVP brain receptors, as foster California males had both fewer and smaller cells at AVP receptors. These results suggest that the road to power can be rerouted when normal developmental patterns are altered.
In the early 2000s, with an understanding of some of the basics of power in both Peromyscus species, Marler, working with a team of students, set her sights on examining winner effects. In these experiments, a male and female were housed together in a cage, and the male was given zero, one, two, or three winning experiences. The winning experiences were provided by allowing the resident male to interact with a smaller, weaker, semisedated male placed in his cage. Next, a healthy intruder, about the same size as the resident male, was placed in the resident’s cage. Marler and her team observed the fights, and when the contests were over, they drew blood samples.
In white-footed mice, although winners had lower stress hormone levels than losers, no winner effects were uncovered; even when a male had just come off three wins in a row, it did not affect his chances of winning against a healthy intruder, and winning had no measurable effect on testosterone levels. In California mice, which rely more heavily on aggression in their quest for power than do white-footed mice, winning matters—but only when it’s winning on a grand scale. Males with one or two prior wins were no more likely to defeat an intruder than were males with no winning experience. But if a California mouse had really been on a roll and had had three wins, he was likely to beat any intruder who entered his domain. This “third time’s the charm” effect was linked to increased levels of testosterone in males coming off a string of wins. Together with two of her students at the time, Matt Fuxjager (now at Brown University) and Elizabeth Becker (now at St. Joseph’s University), Marler was even able to pinpoint the brain circuitry associated with changes in testosterone and winning. But winner effects and the rise in testosterone depend, as many power-related things do, on place and ownership. When Fuxjager and Marler ran a similar experiment but had the males fight outside their territory, the winner effect and associated rise in testosterone were much less pronounced.
Fuxjager and Marler thought more about the differences in winner effects between California mice and white-footed mice, and the role that testosterone plays in mediating these effects. Was it the case that white-footed mice, which showed no winner effects, lacked the physiological machinery to mount a winner effect, or could it be that they have that machinery, but just don’t produce enough testosterone to kick it into gear? They began to wonder what might happen if they experimentally increased testosterone levels in white-footed mice to the levels typically seen in California mice displaying winner effects. Could they mimic the winner effects found in California mice?
Fuxjager and Marler tested 37 white-footed mice in three groups. In one group, males experienced three wins (by being matched against much smaller opponents), and after each win they received an injection of testosterone. Two control groups were also tested. In the first, males that had experienced three wins in a row were injected with saline, to make certain that it was an injection of testosterone, not just any injection, that was causing a winner effect. In the second group, males experienced three wins, but no injections. The testosterone injections appeared to be a power elixir, as white-footed mice that won three times and received testosterone each time showed the same winner effect that had seemed, until that point, to be reserved for California mice.
The extrinsic effects of animals fighting for dominance reach beyond the immediate participants to any bystanders within earshot, which leads us to ethologist Joseph Waas, of the University of Waikato in New Zealand, lying flat in penguin poop night after night in a New Zealand cave.
Waas, a birder to his core, was finishing up his undergraduate work at Trent University, in his native Canada, and was on the hunt for what to do next. John Warham, a pioneer in the study of penguin biology, suggested that Waas check out a colony of little blue penguins (Eudyptula minor) on the eastern side of Banks Peninsula on New Zealand’s South Island. “I went . . . and they just fascinated me,” Waas recalls. “It seemed so cool these penguins lived in caves and in burrow communities and were active at night. And so I started working on their vocal repertoire.”
Joseph R. Waas; Graph from S. C. Mouterde, et al. Animal Behaviour 83.
The smallest of all penguins, standing only about a foot tall, little blues are as cute as can be. But they’re loud. Really loud. “There would be these periods of time,” Waas says, “when all the calling would die down and then one bird or maybe two birds would then call and then you would get this incredible contagious effect until everyone started calling.” His early work was in the cave colonies, where penguins nest about two to three meters apart, usually up against the wall of the cave. Because they are nocturnal, Waas needed to be as well, arriving at the colony at dusk, in time to watch the penguins return from the ocean and waddle to their caves. He’d follow hot on their trail, with a tape recorder and a camera hooked up to a night-vision scope in hand, and stay in the cave with the penguins until 4 a.m. Then back to Christchurch for a bit of sleep, to start the whole round trip over again the next day.
It was not easy work. “The main cave I worked at is in Ōtanerito Bay,” Waas notes. “There are two areas, an upper [cave] area that had maybe a hundred birds . . . and then there was a lower cave, where you had to get on your belly and crawl, which wasn’t very pleasant as the actual base of the cave is made of dried guano and penguin feathers.”
In addition to vocalizations, Waas was also interested in power dynamics in these tiny penguins. Lying on his stomach in the lower cave, what he saw and heard fascinated and frustrated him: birds fighting, interlocking bills, and flipping one another, as Waas says, “almost like a judo throw.” His mind was soon racing with ideas, but how was he going to design an experiment? “What was I going to do?” he says. “I couldn’t set up fights between penguins, so I put it on the back burner.”
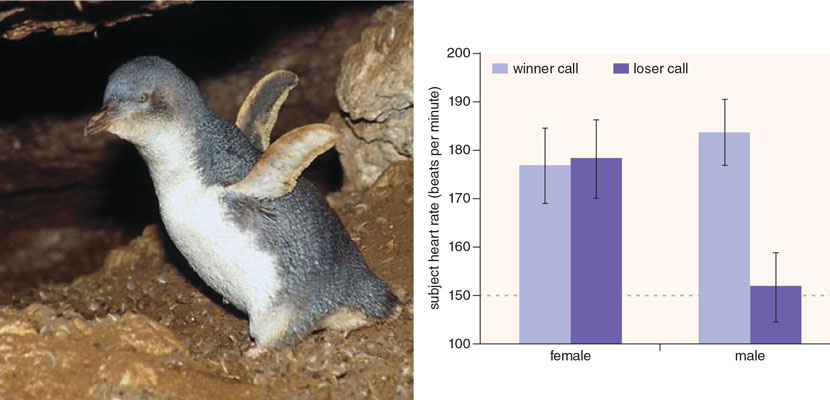
When male little blue penguins heard the triumph calls of fight winners, their heart rates shot up. These eavesdroppers were clearly nervous when those in power were nearby.
One thing Waas could not help hearing over and over on all those guano-soaked nights was the “triumph call” made by males. First described in greylag geese (Anser anser) by ethologist and Nobel laureate Konrad Lorenz, the triumph call in little blue penguins is a high-pitched inhalation paired with a bray-sounding exhalation that is repeated over and over. But it was the context of the call, not its auditory dynamics, that really struck Waas: At the end of an aggressive interaction, the winner often stood up with its flippers extended and “belted out this [triumph display] vocalization,” while “the loser [would go] into a low walk or a low run . . . directly away from the winner.” And sometimes it was doubly spectacular: If a female was on the nest of the male who was making a triumph call, she sometimes joined in.
Waas knew he was not the only one impressed by triumph calls—other penguins, not only the male who had just lost to the caller, were clearly paying attention to these shouts of power as well. Exactly why, and what these eavesdropping penguins might be doing with the information from the call, he didn’t know. Waas began to think he might be able to record these triumph calls and conduct playback experiments in which he could control what was heard so he could study power and possible eavesdropping effects. But the cave he was working in was just too chaotic to control who would hear what, so he turned to another colony near the cave, where penguins lived outside in burrows.
This burrowing colony was in the middle of a farm owned by two of Waas’s friends, Francis and Shireen Helps. “They are alternative farmers,” Waas explains, “and they made a real effort to maintain an area of their farm to protect the colony.” Equally important in the eyes of an ethologist, “they marked all the birds, so for a lot of birds we knew their age and sex, and they also constructed artificial burrows.”
Working with Solveig Mouterde, a veterinary graduate student at the University of Waikato, Waas did just that. They worked with 27 males and 16 females who were incubating eggs alone at their artificial burrows (both males and females incubate eggs) while their partners were off foraging in the ocean. The egg was gently removed and put into an incubator, and an artificial egg was placed in its stead. The artificial egg had sensors that recorded the bird’s pulse and so, indirectly, its heart rate. Next, they put a microphone near the nest they were working with that night, so that they could record the vocalizations of the penguin after it heard a speaker 5 meters away broadcast the sounds of a fight, followed by the triumph call made by the winner or the calls made by the loser of the fight.
When males heard the triumph calls of winners, their heart rate shot up by more than 30 beats per second compared with typical baseline values. No such burst was found when they heard the calls of losers. These eavesdroppers were clearly nervous when those in power were nearby. And they acted like it, too, as they were much more likely to vocalize themselves in response to hearing the sounds of a loser, who would presumably be a weaker potential opponent, than the call of a winner. For their part, females showed increased heart rates when they heard winners or losers, and never vocalized after hearing playbacks, suggesting they were generally agitated by fighting and wanted no part of any of it.
Eavesdropping and audience effects—and, to a lesser extent, winner and loser effects—tell us that experience with others matters in the struggle for power. These extrinsic factors do more than show that size is not everything in the quest for power in animals: They demonstrate the subtle and complex nature of that quest.
This article is excerpted and adapted with permission from Power in the Wild: The Subtle and Not-So-Subtle Ways Animals Strive for Control over Others, published by the University of Chicago Press, © 2022 by Lee Alan Dugatkin.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.