Shedding Light on Dolphins
By David Schneider
Glowing phytoplankton aid in a study of speed
Glowing phytoplankton aid in a study of speed

DOI: 10.1511/1999.24.0
During the early 1970s, a Russian biologist named Y. G. Aleyev set out to explain a longstanding puzzle. Four decades before, James Gray, an English zoologist, had estimated the power that dolphins need to swim (according to some simple principles of fluid mechanics) and compared that figure with what their muscles can generate (according to some simple principles of physiology). Not only did the two numbers not match, but the power requirement was a full seven times the amount theoretically available.
Aleyev hypothesized that fatty skin folds, which form when a dolphin swims rapidly, have special hydrodynamic properties that substantially reduce drag. To test his idea, he assembled a team of professional women swimmers, dragging these volunteers through the water at high speeds either clad in tight-fitting body suits or au naturel. Because these swimmers had about the same thickness of subcutaneous fat as dolphins do, zooming around underwater while in the buff raised similar folds on their bodies. Yet Aleyev's measurements showed that these pleats created more drag, not less, leaving the mystery of dolphin energetics completely unresolved.
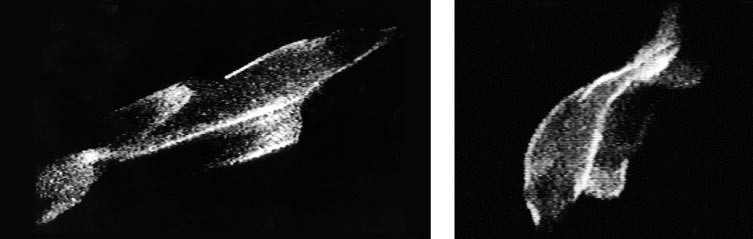
Journal of Experimental Biology, Company of Biologists Ltd.
The persistence of Gray's paradox has more recently prompted Jim Rohr, a civilian physicist working for the U.S. Navy, and Michael Latz, a marine biologist at the Scripps Institution of Oceanography, to once again probe the hydrodynamics of dolphins in a novel way, which they reported last year in the Journal of Experimental Biology. Rather than enlisting the aid of immodest professional swimmers, they received help from some modest single-celled organisms that periodically bloom in the waters around San Diego, primarily dinoflagellates of the species Lingulodinium polyedrum.
It is well known that these marine plankton respond to mechanical stimulation by emitting light. Most reports in the scientific literature had indicated that turbulence is needed to set off the fireworks. But after studying these organisms in the lab, Latz and Rohr discovered that laminar flow too can trigger the bioluminescence, as long as the shear stress (for example, in the boundary layer that forms around a moving object) exceeds a critical threshold.
Armed with this understanding and a low-light video camera, Rohr and Latz boarded a friend's boat one moonless night and set sail into San Diego Bay to see whether they could learn something about the hydrodynamics of dolphins. Although they observed dolphins "lit up" by bioluminescent phytoplankton during their very first outing, the conditions proved difficult to reproduce. Not only must the bioluminescent organisms be adequately abundant and the dolphins sufficiently friendly, but the experiment also requires the darkness of a new moon. And even if nature cooperates fully, "the lights of the city can kill you," notes Rohr.
So Latz and Rohr engineered somewhat more controlled conditions by erecting a large tent over one of the floating pens used for the Navy's marine mammals program in San Diego Bay. With the help of the animals' military handlers, they directed trained dolphins to swim into the darkened pen, where they had positioned their low-light camera.
By recording the ghostly, blue-green bioluminescent halo around the moving dolphins, Latz and Rohr were able to examine the flow of water over the creatures as they coasted beneath the camera. The study revealed that the hydrodynamic boundary layer is typically quite thin up front and that, contrary to expectations based on simple fluid mechanics, the flow does not separate from the body of the animal along its flank.
The surprising lack of flow separation (and the concomitant drag that separation would cause) may help to explain why Gray's calculation was in error. But the full solution may in fact be more prosaic. "The simple answer is that they're not moving as fast as everyone said," says Frank Fish, a zoologist at West Chester University in Pennsylvania.
Gray had supposed that dolphins could swim at 20 knots, whereas modern estimates indicate that dolphins cannot sustain much more than 15 knots. Because the energy expended underwater scales with the cube of the speed, this difference alone more than halves the theoretical energy requirement. Other simple differences, for example between the muscles of dolphins and the muscles of the human athletes on which Gray based his calculations, can also help reconcile the discrepancy. But a major question still remains: Why does water flow smoothly over the body of a dolphin without separating in the way that simple hydrodynamic theory predicts? Fish is eager to join with Rohr in solving that riddle using natural bioluminescence, but he is guarded about their prospects, given the many experimental difficulties involved: "If we have some money," says Fish, "and the planets are aligned correctly, maybe we can pull it off."—David Schneider
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.