One Shocked Chemist
By Roald Hoffmann
Molecular surprises are sometimes right in front of us, if only we’d do the math
Molecular surprises are sometimes right in front of us, if only we’d do the math

DOI: 10.1511/2011.89.116
This is the story of a theoretical result that astonished me. But it shouldn’t have.
A chemist for nigh on 50 years, I’ve had the joy of seeing lots of new molecules in the literature. Let me show you a couple, just so you see what surprises me.
Some new molecules are striking, just because they are extensions of familiar motifs that no one previously thought of changing. Take the “N-confused” porphyrins, compounds with a fitting name that tells us something about our history, expectations, and the nice informality of chemical nomenclature. Porphyrins (top of first figure, below) are a classic group of molecules in which four so-called pyrrole rings—five-membered rings with four carbons and a nitrogen—face their nitrogens toward an interior void that is often neatly filled by a metal atom, for instance an iron in the heme unit of hemoglobin. In the N-confused porphyrins (bottom of first figure, below), one pyrrole ring is turned, and a carbon atom faces the center, not a nitrogen atom. It makes a difference, of course. And I’m glad someone thought of ringing the changes on a classic motif. N-confused porphyrins were first reported by two groups in 1994, those of Lechoslaw Latos-Grazynski at the University of Wroclaw in Poland, and of Hiroyuki Furuta of Oita University in Japan.
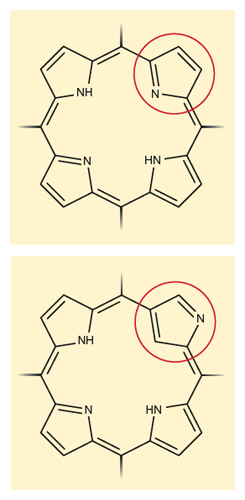
Illustration by Tom Dunne.
Some new molecules are simply astounding. We have learned that xenon, a so-called noble gas, is far from inert, forming bonds with halogens, oxygen and carbon. But I never imagined a bond between two relatively unreactive elements, gold (Au) and xenon (Xe), and, to boot, with a pretty naked xenon acting as a ligand—which is usually an ion or a molecule that binds to a central metal atom by donating one of its electron pairs. But that’s what Stefan Seidel and Konrad Seppelt of the Free University of Berlin made in 2000 in the square-planar AuXe42+ ion shown in the second figure.
Let me tell you a story here of another molecular surprise that recently came my way. In the process, I was led to reflect again on the relation between stability and existence in chemistry (as I wrote about in “Unstable,” American Scientist November–December 1987).
Xiao-Dong Wen, a talented postdoc in the small group that Neil Ashcroft and I have at Cornell University, has been studying benzene under pressure. People have been squeezing benzene between diamond anvils for some years, eventually obtaining some amorphous polymeric material, not terribly well characterized, in which carbon-carbon bonds have formed. We have been (and still are) looking theoretically for a hypothetical metallic benzene, perhaps one in which the scales of mobility of electrons, protons and carbons are separated.
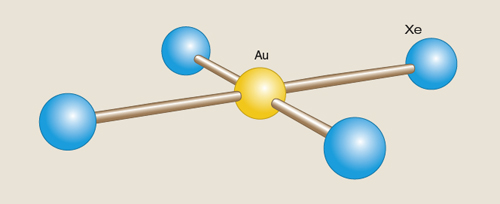
Illustration by Tom Dunne.
At a certain high pressure, Xiao-Dong found that benzene rearranged spontaneously to not one, but a family of regular two-dimensional polymers of unchanged composition, CH, the same as benzene, C6H6, or (CH)6. But the polymers Xiao-Dong found, unlike benzene, are not aromatic, meaning that they are not especially stabilized (the way that benzene is) by a hexagon of alternating single and double bonds between the carbon atoms. The 2D networks are shown in the third figure; they would today be called graphanes.
The 2010 Nobel Prize in physics was given to Andre Geim and Konstantin Novoselov, both of the University of Manchester in England, for the isolation and characterization of graphene. The sheetlike structure of carbon in graphite has been long known; the honeycomb sheets of carbon are loosely bound to each other by weak intermolecular forces. But it took the courage of a simple physical procedure, drawing a block of purest graphite across a piece of adhesive tape (technically, “exfoliation of pyrolytic graphite”), for Novoselov, Geim and their coworkers to make reproducibly isolable and manipulable multilayer and monolayer sheets of graphene.
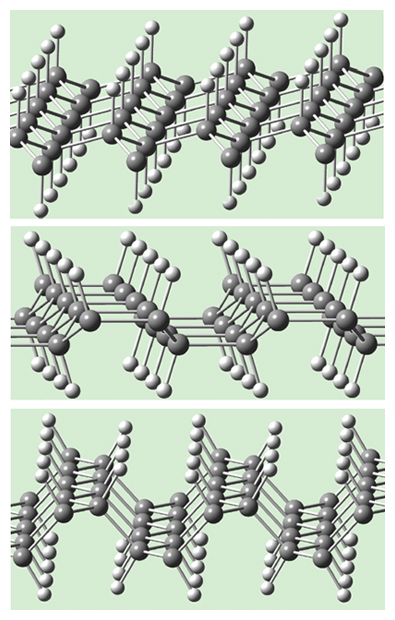
Illustration by Roald Hoffmann.
Graphane is a single layer of graphene that is hydrogenated, with one hydrogen added per carbon in the graphene layer. If the hydrogens are added regularly above and below the sheet at alternate carbons, one gets what a chemist would call all-chair cyclohexane rings. One can also get a variant with boat cyclohexane rings, as shown in middle of the third figure (above), as well as a third one, in the bottom of the third figure, whose structure is related to a common motif in hundreds of inorganic compounds (such as BaIn2 or TiNiSi). (A fourth variant, also built from boat-shaped rings, has been added for this online edition [below]).
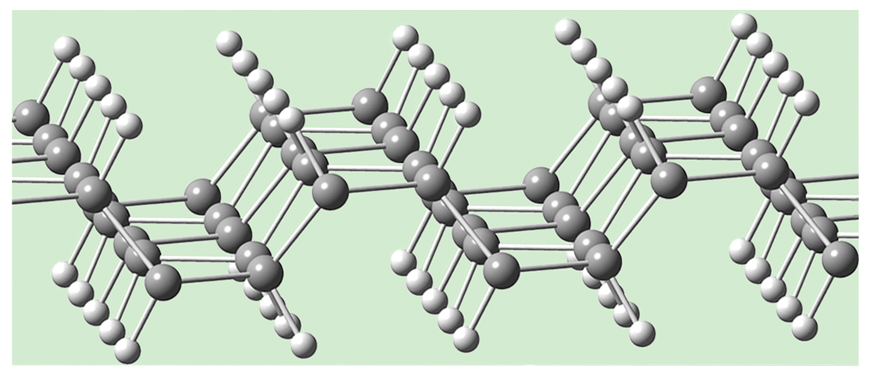
Graphene is real. But has graphane been made? The approach of Daniel C. Elias and his colleagues at the University of Manchester comes closest, but we have no crystal structure, only rough images of the material from transmission electron microscopy. To be sure, there is no shortage of theoretical calculations of graphane.
Xiao-Dong found that over a wide pressure range, all three graphanes were more stable thermodynamically than benzene, per CH. When he showed me his computational results, I immediately said, as all research advisors do, “There’s something wrong with your calculations.” When he demonstrated to me that his calculations were correct, I sat back for a while and closed my eyes.
As a chemist, I have an intuition honed by 50 years of practice. It’s a pressure = 1 atmosphere intuition, of course. Under Neil Ashcroft’s tutelage, I have learned recently of the strange world of high pressure.
But a CH hydrocarbon that is more stable than benzene at ambient pressure? Benzene is the prototypical aromatic molecule, the emblem of chemistry. The six-carbon ring is a conserved entity in the chemical universe—one can run a multitude of substitutions on the ring, replacing one hydrogen after another. So from benzene, one can go (and chemists already did in the 19th century) to chlorobenzene (C6H5Cl), to toluene (C6H5CH3), to nitrobenzene (C6H5NO2), to the explosive trinitrotoluene (TNT, C6H2(CH3)(NO2)3), to aspirin, mescaline, novocaine, estrone… any of a host of substituted benzenes. All the time, in biological systems as well as the laboratory, the hexagon of carbons remains intact, a testimony to its stability.
Benzene is such a good thing that the literature is in fact full of mostly imagined “aromaticities,” supposed harbingers of stability that exist only in the paper-writers’ hype-addled minds. But the stability of the parent C6H6 system is sacrosanct. It’s hard to argue with it.
Except that benzene is unstable relative to graphane. Our calculations were showing this. And I should have known it.
Let me tell you why I should have known it. The fourth figure (below) imagines a hypothetical formation of graphane from an array of benzenes. In the first step, the benzene molecules are deprived (on paper) of their aromaticity, their bonds localized into the cyclohexatrienes that August Kekulé (a principal founder of the theory of chemical structure in the mid-1800s) struggled with conceptually. How much might this “loss of resonance energy” or “loss of aromaticity” cost? (See the reference to the paper of Shaik and his coworkers for a clear discussion of different definitions of resonance energy.) One estimate is about 270 kilojoules per mole.
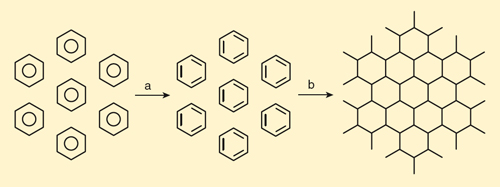
Illustration by Tom Dunne.
In the next step, the “dearomatized’ benzenes polymerize into a two-dimensional sheet. In the process six carbon-carbon sigma (σ) bonds (the strongest type of covalent bond) are formed, although there are effectively three per ring, because each new bond is shared by two benzene rings. Three double bonds in each cyclohexatriene are converted to single bonds.
What is the relevant energetics for the second step? The second (π) bond of any double bond is worth less than a single bond. This energy is not a number one can measure directly. One can get an estimate from the heat of reaction of three ethylenes (C2H4) to cyclohexane (C6H12), a process in which three π bonds are converted to three σ bonds. That heat is experimentally –282 kilojoules per mole. Another way to estimate the energy of breaking a π bond is to look at the energy of rotating the two CH2 groups in ethylene 90 degrees out-of-plane, and to compare that to the strength of a C-C σ bond. That would lead to a slightly larger estimate of –315 kilojoules per mole for three bonds.
The sum of the two heats for the processes is +270 –282 (or –315) = –12 (or –45) kilojoules per mole for C6H6. There are all kinds of assumptions being made here, and I’ve neglected the obvious entropy change in the process, favoring benzene. But the essence staring us in the face, what I should have seen but didn’t, is that graphane is more stable than benzene.
That conclusion is what the better calculations give too. The number varies with the quantum-mechanical method used; we get that all-chair graphane is about 90 kilojoules per mole of C6H6 more stable than benzene. I should mention that we were not the first to calculate that graphane is more stable than benzene. That was done by Jorge O. Sofo, Ajay S. Chaudhari and Greg D. Barber of the Pennsylvania State University. Perhaps I was the first to be surprised at the result, with the baggage of chemical experience weighing on me.
If something is thermodynamically stable, one should be able to make it. Yes, but….
What matters in chemistry is not thermodynamic stability, but kinetic persistence. Chemistry is the land of thermodynamically stable or (more interesting) unstable molecules that have high barriers to going to where they (or we) want to go. For example, nearly every molecule in our bodies—with the exception of H2O, CO2, phosphate and some other small ions—is thermodynamically unstable in the presence of oxygen. Were it not for the water in us, and the high barriers to oxidation, we should burn very nicely. Literally, not just with passion.
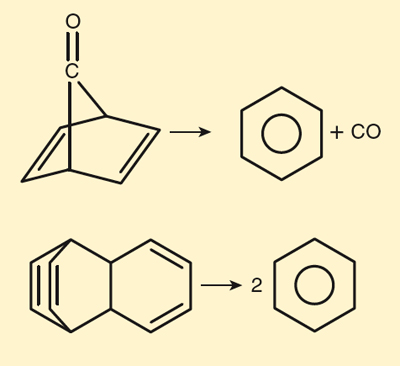
Illustration by Tom Dunne.
Another example: Of the four most stable (with respect to separation into atoms) diatomic molecules that are made of identical atoms—N2, C2, P2, O2—only two are available in a bottle at room temperature and pressure. Whereas other diatomics, much less stable to atomization, are there at ambient conditions—F2 and Cl2 for instance.
Additionally, benzene itself has a positive heat of formation from the elements, graphite and diatomic hydrogen. It shows no hint of decomposing to them, of course. The barriers to initiating that decomposition are stupendous.
Graphane is a two-dimensional raft of CHs. Organic chemists, masters of parlaying designed complexity in zero-dimensional molecules into other zero- and one-dimensional molecules, have trouble exercising control in two and three dimensions. There are emerging exceptions, for instance in the self-assembled complexity of metal-organic frameworks.
Graphane is slightly more stable than benzene, but there is no systematic, bond-by-bond route to graphane (nor was there to buckminsterfullerene). Elias and his colleagues, in their approach to perfect graphane, reacted a suspended graphene sheet with a plasma of hydrogen.
The reactions forming graphane from benzene in the fourth figure were a Gedankenexperiment, not a synthetic route. To be sure, they are all “allowed,” in the sense that Robert B. Woodward and I delineated some years ago. But entropy factors aside, they are nevertheless certain to have substantial barriers to proceeding—bonds have to be broken partially in the initial stages of the reaction, and that costs energy. So, a Diels-Alder cycloadditions of ethylene and butadiene to cyclohexene, a prototype-allowed reaction, still has an activation energy of 115 kilojoules per mole. The fifth figure shows two allowed fragmentations that are highly exothermic, yet have energy barriers of about 65 kilojoules per mole. Benzenes are very, very unlikely to form graphane in the way the fourth figure shows.
I am sure pure graphanes will be made, in some nonsporting yet reproducible way. I use the plural, because the three structures drawn above are stereoisomers (they differ in the three-dimensional arrangements of their atoms in space) and although not very different in energy, face gigantic barriers to conversion amongst themselves. And, once made, graphanes will not decompose spontaneously to benzene. Even as kinetics is what matters, thermodynamics rules.
I am very grateful to Sason Shaik for correcting some of my thinking, to Jerome Berson for a comment and to Xiao-Dong Wen for his research and illustrations.
©Roald Hoffman
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.