Microsized Architecture
By Robert Frederick
Three-dimensional printing is now at capillary sizes, for better modeling of living systems.
Three-dimensional printing is now at capillary sizes, for better modeling of living systems.

No known structures in the human body look like this swirling set of tubes (below). But the capacity to create them with an inner diameter smaller than 10 micrometers is critical to making better models of the body’s structures. Researchers use such models for a wide variety of biomedical purposes, including pharmaceutical testing. But modeling capillaries, for example, which vary between 5 and 10 micrometers and withstand significant fluidic pressures, is a challenge.
“To my knowledge,” says Ryan Sochol, “our approach offers the only way to create microfluidic tubules or vessels that have circular cross sections [as capillaries do] and tortuous architectures in the sub-100 micrometer range.”
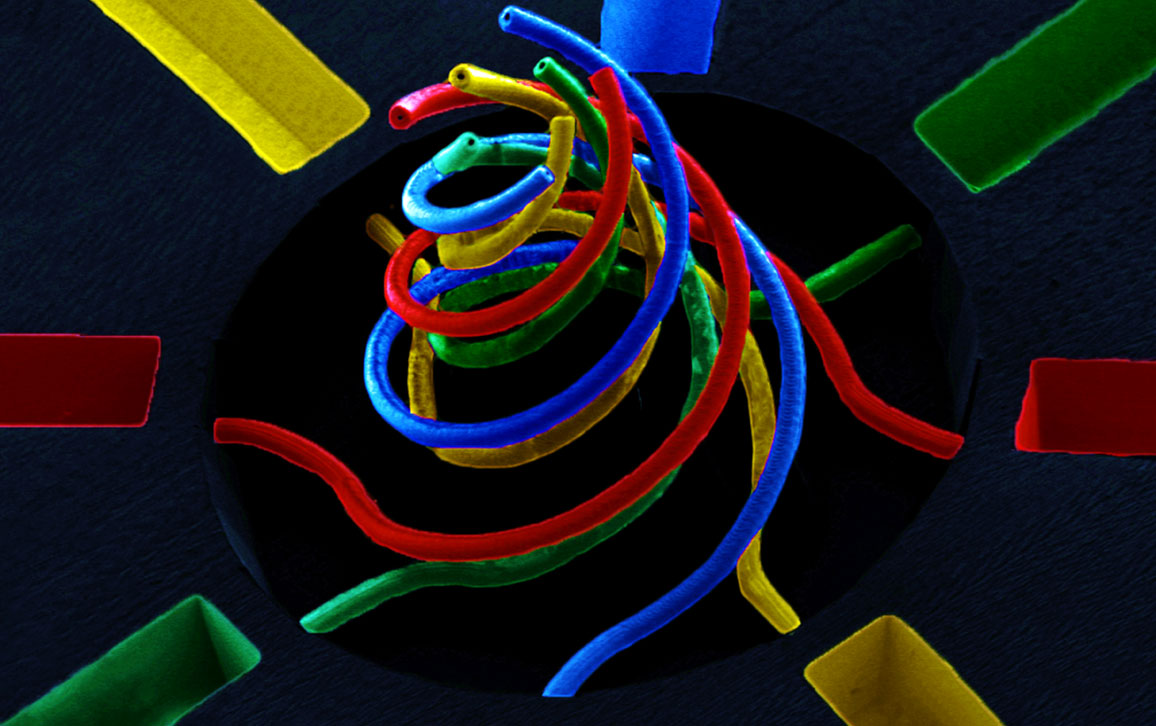
Sochol is a University of Maryland engineer whose team made this order-of-magnitude fabrication improvement with a type of three-dimensional printing called direct laser writing. His team published on the technique earlier this year in Scientific Reports and twice in Lab on a Chip. Direct laser writing uses special liquids that cure, or polymerize, when struck with light. Making sub-100 micrometer devices required femtosecond (one quadrillionth of a second) laser pulses, which limit the number of photons striking and hardening the material. “Afterward,” says Sochol, “you get rid of the liquid, and the only thing that remains is the hardened object.”
Washing in successive liquids and curing them one by one enables Sochol and his team to create microdevices made from multiple materials, as this scanning electron microscope image depicts (it is falsely colored to highlight multiple material types). “The printing process is completely dependent on what you’re making,” says Sochol, “but most of our prints take on the order of 10 minutes to an hour, and that’s where we try to keep it” because longer printing times quickly become costly and wasteful.
The remaining challenges were connecting these tiny devices to larger ones in order to deliver fluids and having these devices withstand the fluidic pressures necessary for testing. Sochol’s team solved the pressure problem by switching from a commonly used silicon-based organic polymer (polydimethylsiloxane, or PDMS) to a less common but increasingly used one called cyclic olefin polymer. To solve the connection problem, the team built the larger, fluid- delivering structures first using more traditional methods and then used direct laser writing to create—and bond—the smaller structures inside them.
About collaborating with his university’s Institute for Biomedical Devices, Sochol says, “Our end goal is to create living systems into which we load different types of cells to learn more about how they interact.” The team’s next step is to ensure that they can adapt their processes to make devices with environments suitable for living cells and tissues.
An audio interview with the researcher:
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.