Is Garlic Mustard an Invader or an Opportunist?
By Michael Anderson
Originally thought of as simply harmful to native plants, this invasive herb has been spreading for far more complex reasons.
Originally thought of as simply harmful to native plants, this invasive herb has been spreading for far more complex reasons.

The first time I saw garlic mustard I did not know enough to be alarmed. I first noticed the plant early in the spring of 2005, while wandering the grounds of my new home at Macalester College’s Ordway Field Station in Minnesota, where I had just been hired as the resident caretaker. The plant was one of the few green things in the forest at that time of year, but I could not find it in any of the station’s field guides.
The following summer, I learned from local botanist Karen Schick of the Friends of the Mississippi River that the plant was garlic mustard (Alliaria petiolata). Visibly concerned, Schick informed me that the plant was invasive in Minnesota. Garlic mustard lives for two years, with two distinct growth stages. The greenery I had first noticed were patches of yearlings called rosettes, a low-lying form that sprouts from fresh seed and remains green all winter. By June the following year, these had grown into thick stands of chest-high adults that looked like an invasion to me, now that I knew what I was looking at. Few native plants were evident between the tall stems, as if they were falling beneath an advancing foreign army.
Stephanie Freese
A few years after my initial encounter with garlic mustard, I began to work with two Macalester professors—Mark Davis, the well-known invasion ecologist who spearheaded our research program, and Jerald Dosch, as well as dozens of gifted undergraduates—to learn about the connection between garlic mustard and native plant communities. Since then, garlic mustard has become one of the most intensively studied invasive plant species in the United States. The picture that is emerging from all this work provides an unusually detailed case study in the intellectual hazards of ecological research. Every aspect of the “invasion” of this species has turned out to be richer and more complex than it first seemed—regarding both its causes and its consequences, and even the term invasive itself.
The invasion of garlic mustard into my backyard forest is the tiny tip of a large iceberg. Globally, approximately 14,000 plant species have become naturalized, meaning plants that humans introduce to a new region that then form self-sustaining populations. Of these species, a smaller and more difficult to define fraction—perhaps 2,500 species—have become invasive, meaning they have grown numerous enough or they have large enough per-capita effects for them to be considered pests.
The term invasive is controversial, due to wide variation in its intended meaning, not to mention its xenophobic and militaristic connotations. I use the term here because it is the most familiar and widely used descriptor of such species. To be clear, I use the term in the restricted sense established in President Barack Obama’s 2016 executive order on invasive species: “a non-native organism whose introduction causes or is likely to cause economic or environmental harm, or harm to human, animal, or plant health.”
Determining whether any given nonnative plant species is likely to be harmful enough to qualify as invasive is far from straightforward. The ecological and economic effects of such plants are notoriously difficult to assess and are controversial even among the scientists who study them. Estimates of the economic costs run into the billions of U.S. dollars. Invasive plant species are also often associated with diminished diversity of native plant communities and changes in the function of their ecosystems. However, determining the cause-and-effect relationships of such associations is one of the trickier aspects of studying species invasions. Garlic mustard, considered invasive not only in Minnesota but also in the entire northeastern quadrant of the contiguous United States, is an excellent example of the difficulties involved.
Early field surveys noted garlic mustard’s tendency to form dense stands that contained low numbers and diminished diversity of native plants. Experimental studies conducted during the same period discovered that garlic mustard produced a variety of toxic compounds that wound up in soils inhabited by the plant. In laboratory experiments, these compounds inhibited the growth of fungi, including the kinds that form beneficial mycorrhizal symbioses with the roots of most plant species. Garlic mustard, as a member of the mustard family, is one of the minority of species that does not participate in these symbioses. Therefore, it seemed likely that the “chemical warfare” waged by these compounds—an interaction known as allelopathy—probably allowed it to muscle its way into intact native communities. If that was the case, it also seemed likely that the plant was poised to cause significant damage to native ecosystems.
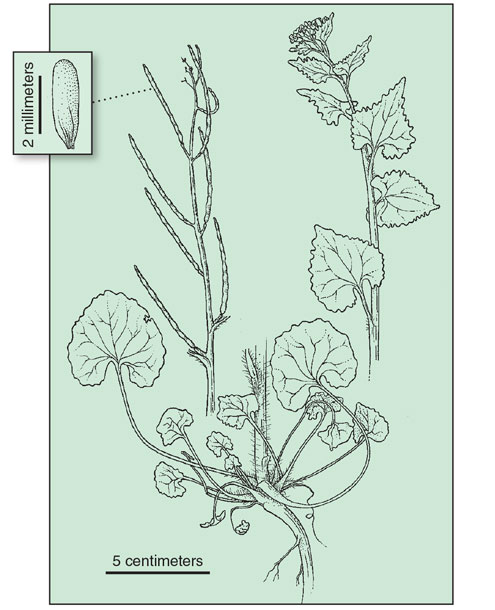
Douglas, G. W., et al (Editors). 1998. Illustrated Flora of British Columbia, Volume 2 © Province of British Columbia
Invasive plants are often assumed to spread by outcompeting native species. In garlic mustard’s case, this hypothesis is certainly plausible. The adult plants are tall, compared to other forest-dwelling herbs, which provides a significant light-gathering advantage in dim understory environments. The rapid growth of adults in their second year of life also seems likely to deplete soil resources such as water and nutrients, lowering their availability to smaller native plants. And garlic mustard’s possession of allelopathic compounds gives it a novel weapon in its struggle for existence with native plants. If garlic mustard owes its success to such advantages, then—to borrow an analogy developed by Andrew MacDougall of the University of Regina and Roy Turkington of the University of British Columbia—it could be “driving” the ecosystem changes that seem to be associated with it. In other words, garlic mustard could cause diminished abundance or diversity of native plants by directly harming them.
This possibility has caused a great deal of concern among researchers, land managers, nature enthusiasts, and landowners in states across the plant’s naturalized range. Descriptions of garlic mustard in informational materials provided by land management organizations often include such terms as “aggressive,” “highly invasive,” and “destructive,” and commonly reference its superior competitive ability and allelopathy. Such organizations often arrange volunteer events billed as “garlic mustard pulls” that aim to “clean up” “infested” areas by hand-pulling garlic mustard plants by the thousands. In 2013, the state of Minnesota added garlic mustard to its list of prohibited noxious weeds, citing competitive ability and allelopathy in its description of the effects associated with the plant.
However, things are not always as they seem, especially in ecology. As garlic mustard research has expanded and diversified over the past decade and a half, the simple cause-and-effect scenario that initially seemed so clear—and so alarming—has begun to yield to a picture that is more complex. Further work suggests that garlic mustard is not quite the superior competitor it appeared to be, and that its spread may have as much to do with large-scale environmental changes as with characteristics of the plant itself.
If garlic mustard is an aggressive spreader able to outcompete its way into intact ecosystems using chemical weapons, one puzzling question is why it has taken so long to spread. Garlic mustard seems to have been introduced by settlers to the United States in the mid-19th century, probably for culinary use: The earliest known record of its occurrence is from Long Island, New York, in 1868. By 1918, herbarium records indicate that it had arrived in Illinois. However, it was not until the late 1980s that its rate of spread had increased enough for it to be seen as a potential problem in the United States. Why the delay?
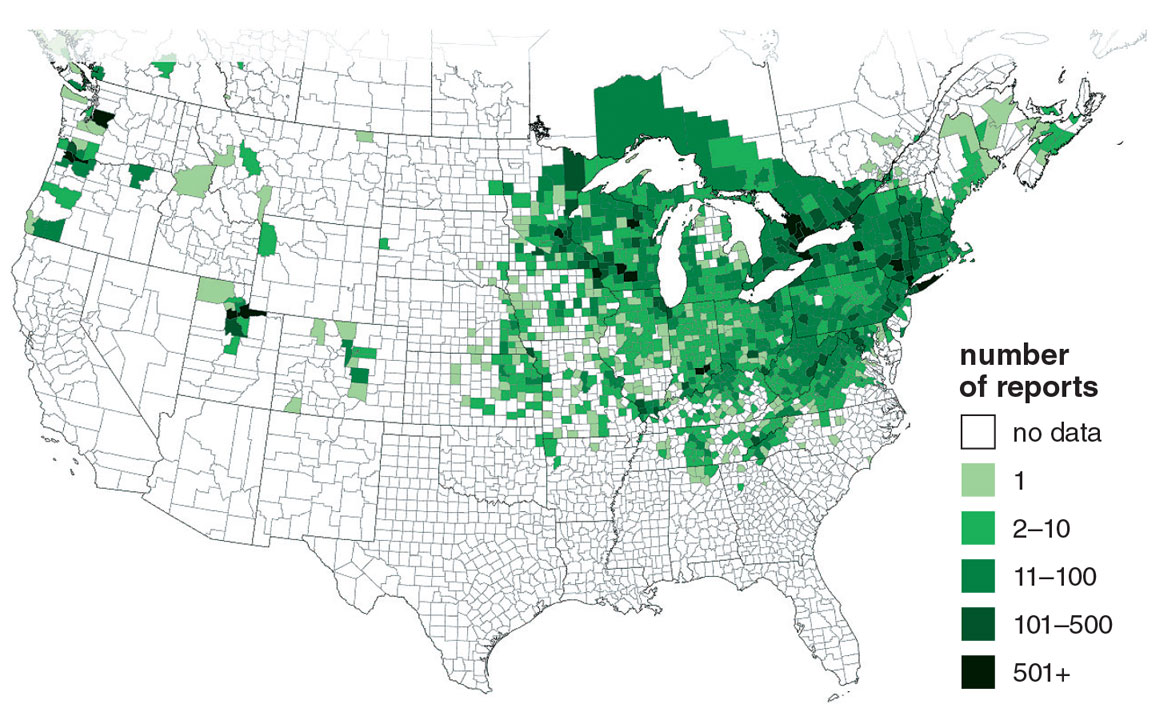
USDA / eddmaps.org
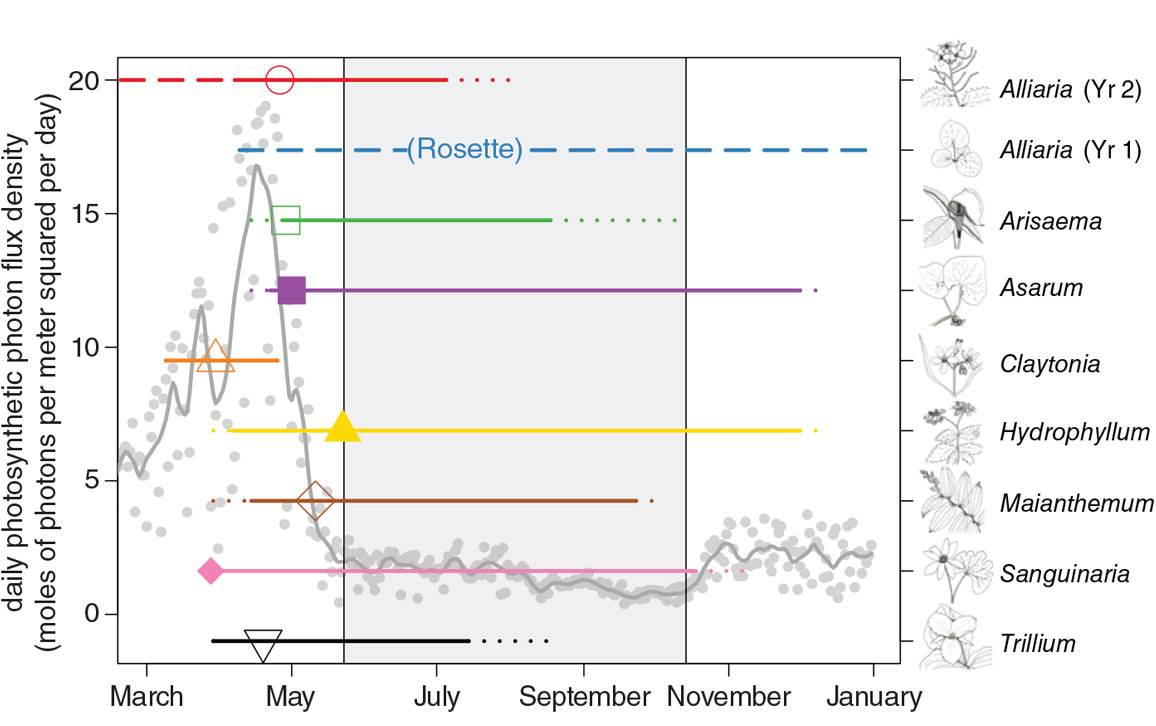
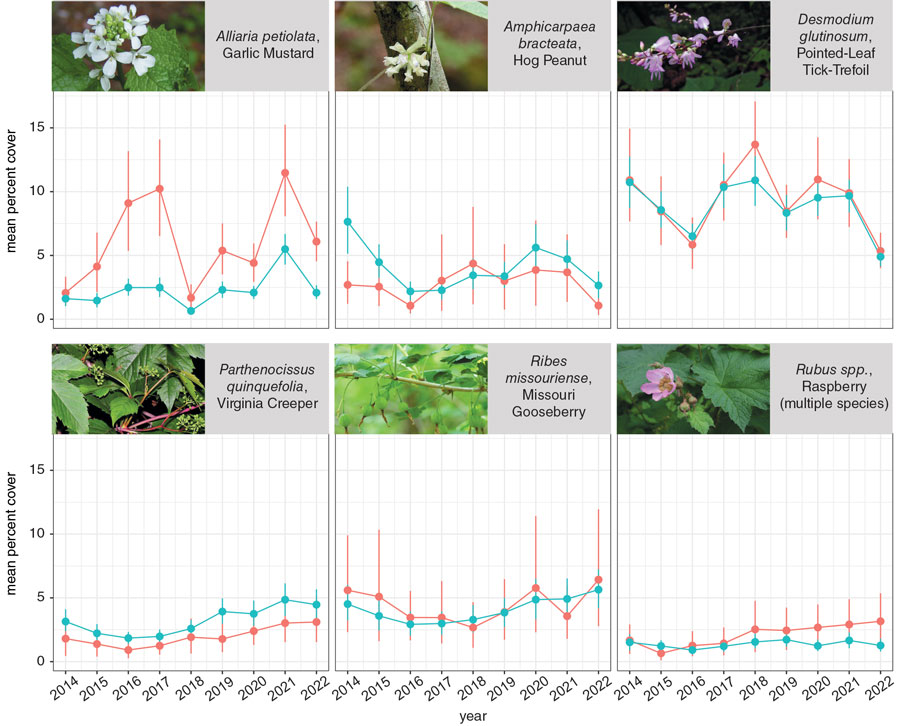
Another inconsistency in the garlic-mustard-as-superior-competitor scenario is the fact that continuing field surveys have failed to observe the clear negative impacts on native communities that were initially expected. At Ordway, looking for such impacts has been one of our main objectives. We have observed the understory plant community in our oak forest in a set of 180 research plots every year since 2014. When we began the experiment, we expected that if garlic mustard were a strong enough competitor to be driving ecosystem changes, we should see stronger or more consistent negative correlations between garlic mustard and native species than we saw between native species. For all species, we measured both the size and number of plants in our research plots using the percentage of each plot’s area covered by a given species. For native species, we also measured diversity, the number of native species present in each plot.
One difficulty with studying plants in the forest understory is that there are patches of ground full of plants and others where plants are sparse. Because of the low light available in most locations across the forest floor, plant density tends to be low in a large fraction of research plots that are placed at random or regular intervals—so low, in fact, that plants in such plots are unlikely to be competing at all. Plant densities high enough to potentially trigger competition tend to occur in areas with high light availability, such as a gap in the forest canopy caused by a recent tree fall. To account for this difference and maximize our chance of finding any evidence of competition that might exist, we analyzed plots with high or low plant density separately.
In the high-density plots, garlic mustard was negatively correlated with abundance and diversity of native plants, especially woody species. However, the strength of the correlations and their consistency across years were similar to those of the most common native species at our site. Thus, if the negative correlations are due to competition (an additional wrinkle not discernible from our field data), garlic mustard’s strength as a competitor is about what we would expect from a native plant that is as common as garlic mustard. Our results agree with continuing field studies that have found negative associations between garlic mustard and native plants only uncommonly. Even when they have been found, those associations tend to be weak or inconsistent over time.
Garlic mustard’s lack of competitive dominance in the field makes sense in light of further laboratory studies of its antagonistic abilities. Head-to-head competition experiments, which compare the growth of garlic mustard and other plant species when grown alone versus together in the same pot, have found that garlic mustard is not the overwhelmingly dominant competitor it was initially feared to be. Some native species do seem to get beaten out in such trials, based on how strongly they are inhibited by garlic mustard and how strongly they inhibit the invader in return. However, many native species seem to be a relatively even match, and a few even outcompete garlic mustard.

Jim West/Alamy Stock Photo
More detailed studies of garlic mustard’s allelopathy have also revealed that production of the toxic compounds varies widely among garlic mustard populations, environmental conditions, and even season, and that native plant species differ widely in their susceptibility to allelopathy. Garlic mustard also has self-inhibitory effects. Competition from adult plants strongly inhibits the smaller rosettes, particularly in dense populations. The effect is strong enough that it may even be driving the evolution of weaker competitive abilities in the species, in order to diminish the inhibitory effect of adults on their own offspring.
Native plant communities may also evolve in response to garlic mustard’s presence. Both native plants and their mycorrhizal fungi appear to develop resistance to garlic mustard antagonism that increases with their time of exposure to the invader. This development of resistance even seems to be driving a coevolutionary response in garlic mustard. Tissue concentration of allelopathic compounds diminishes from younger to older populations of the invader in North America, likely due to the cost of producing them and the diminished benefits they yield in more resistant native communities. The growth rate of garlic mustard populations also appears to diminish the longer the species is in a given location, perhaps due to such decreased competitiveness or to the evolutionary “discovery” of invasive populations by North American soilborne pathogens and parasites. The upshot of all this further work seems to be that garlic mustard is less of a competitor than it seemed at first, and that its competitive ability is likely decreasing over time.
If garlic mustard invasion is not due to the plant’s superior competitive ability, quite a lot of alternative explanations are plausible. Invasion biologists have developed so many hypotheses to explain species invasions that another focus within the discipline has been classifying these hypotheses into broader conceptual frameworks. Continuing work with garlic mustard has shown that a large fraction of these alternative hypotheses are also likely to help explain the plant’s spread. Many of these alternatives would put garlic mustard in the role of “passenger” rather than “driver” of ecological change. In other words, the spread of garlic mustard might be an effect rather than a cause of changes that are occurring in native ecosystems. The plant may largely be riding on the coattails of favorable environmental changes driven by humans.
Both native plants and their mycorrhizal fungi appear to develop resistance to garlic mustard antagonism.
The North American environment to which garlic mustard was introduced in 1868 is not the same environment across which its population has been recently expanding. In the past century and a half, human populations in garlic mustard’s invaded range have exploded in number, carved our way deeply into the pre-European landscape, and changed our technology and lifestyles in ways that have increased the environmental footprint left by each one of us. These human-made changes have ripple effects in the ecosystems associated with us—affecting even native species—favoring some organisms and disfavoring others.
At the same time, growing human-generated carbon dioxide emissions have not only increased the atmospheric concentration of this important plant resource, but also the average global temperature by more than 1 degree Celsius. Temperature increases have been even greater in garlic mustard’s invaded range—more than 1.7 degrees Celsius in Minnesota, for example—and have brought with them altered patterns of seasonal temperature, precipitation, and an extended growing season. Any of these factors could be involved in garlic mustard’s invasion.
The success of any species in a particular place is a complex function of its history there, its inherent traits, and the qualities of the environment around it. Invasive species often seem to possess traits that the native species lack and that allow them to interact differently with the environment. Looking for such differences has been another major focus of research on garlic mustard, including ours at Ordway. The picture emerging from our work and other research in our field suggests that garlic mustard differs in many ways from common native plants. However, these differences may be especially favorable for invasive spread following recent, human-caused environmental changes. There are many such changes, and the spread of garlic mustard may require more than one.
One way garlic mustard differs from the native herbs in North American forest understories is in its seasonal timing. Garlic mustard utilizes a strategy called extended leaf phenology, which means that it is green and active during portions of the year when most native species are dormant. Nearly all the native herb species in Ordway’s forest have a deciduous seasonal strategy. They lose their leaves and stems in the autumn, spend winter protected in the soil as some form of dormant rootstock, and then reemerge the following spring after the worst of winter has passed.
Garlic mustard’s extended phenology derives from its two-year life cycle. The first-year rosette stores much of its photosynthetic product below ground rather than using it to grow. Its above-ground tissues remain green through winter. The following spring, the rosette taps its stored resources to grow into a tall, leafy adult that emerges before the leaves of the canopy trees. At Ordway, adults flower by mid- to late May—earlier than most native species—and pollinated adults fill seed pods and begin to die back by mid-July, before growth has peaked or flowering has begun for most of the native species.
Leafing out earlier in the year than the canopy trees and retaining active leaves later in the year gives garlic mustard access to more total light over the course of the year than the deciduous native herbs receive. Its activity during the spring and fall “shoulders” of the growing season also provides access to more intense light than is available closer to the middle of the season, when the dense canopy of the trees plunges the understory into deep shade. Earlier spring emergence gives garlic mustard access to more of the pulse of water and nutrients made available by melting snow and thawing soils, and decreases its exposure to competition for these resources. The potential advantages provided by extended leaf phenology appear to be great enough that a high proportion of other invasive plant species in North America seem to possess some form of it.
One of the most common large herbivores in American deciduous forests, white-tailed deer, avoids garlic mustard.
Although the hypothesis that extended leaf phenology aids invasion in North American forests makes sense, it also has a few issues. One is the obvious follow-up question: If extended leaf phenology is advantageous for invaders of North American forests, why has it not evolved in native plants? A likely answer is that the advantages provided by extended leaf phenology are contingent on recent changes in the North American climate. Many invasive plants with extended leaf phenology evolved the trait in their home ranges of Europe and Asia, where the climate during the shoulders of the growing season has historically been more predictable from year to year than in North American deciduous forests. Their success in their new home may indicate that the North American climate is becoming more similar to that of their Eurasian homelands. A second issue is that many plants with extended leaf phenology that have been imported to North America from the same home range have failed to become invasive. So even if the match between extended leaf phenology and climate change contributes to invasion, it cannot be the only factor. As it turns out, several other unusual traits of garlic mustard may aid in its recent spread in North America, as they interact with additional human-induced changes.
Garlic mustard also seems to differ from native herbs in its interaction with herbivores. For those who have not had the pleasure, the name garlic mustard is a surprisingly accurate description of the plant’s odor and flavor. I can personally attest that its leaves make a nice base for pesto and a tasty addition to salad. Its odor and flavor derive from a class of sulfur-containing compounds that permeate the tissues of most species of the mustard family. Ironically, the likely purpose of these compounds is to produce the opposite effect they have on humans: to make animals less likely to eat the plant. These compounds appear to work quite well. Garlic mustard has few natural enemies, particularly in its invaded range.
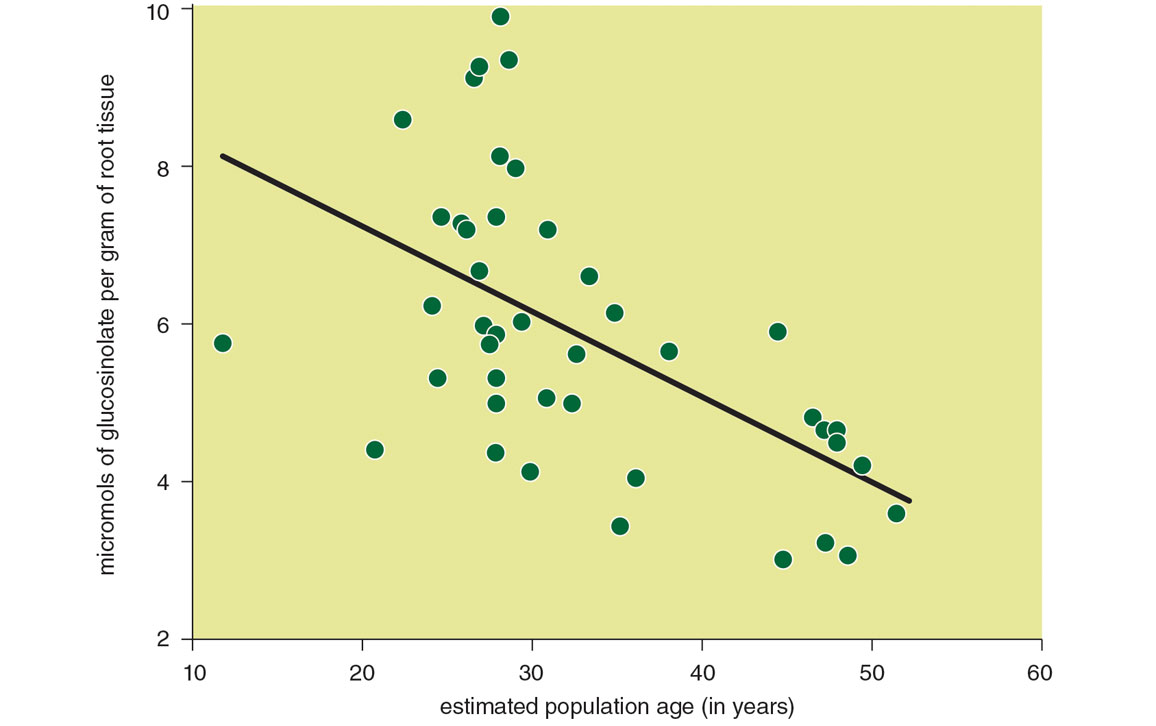
R. A. Lankau et al., 2009
One of the most common large herbivores in American deciduous forests, white-tailed deer (Odocoileus virginianus), avoids the plant. Deer’s aversion to garlic mustard has been widely noted across its invaded range. At Ordway, this behavior is also apparent: In four years of detailed surveys, we have only observed deer herbivory on four individual plants, despite the fact that garlic mustard is the second most common species in the understory. The deer do eat native species, in high quantities and in rough proportion to their occurrence. This selective feeding may clear the way for garlic mustard to spread.
Even if this selective feeding helps drive garlic mustard invasion, however, the scale of the plant’s resulting spread may once again ultimately result from recent human-induced changes. In contrast to many species that have declined with human developments, white-tailed deer populations have expanded alongside them. Humans have removed or greatly diminished many of deer’s natural predators such as wolves and coyotes. The habitats we love to create for ourselves—especially agricultural and suburban landscapes, with lots of food crops and oversized flowers and fruits—are also the favorites of white-tailed deer. Deer are now considered overabundant in many parts of their range, including Ordway, where the Minnesota Department of Natural Resources has estimated that there are more deer than available resources can support in the long term. Due to their large size, rapid reproduction, and voracious consumption, deer overabundance has a wide range of direct and indirect ecological effects. As their populations have expanded with human-altered habitats in recent years, it seems likely that their selective avoidance of garlic mustard can help explain the expansion of the plant.
One of the most striking ways to test this hypothesis is by using exclosures: fences that keep deer out of specific areas. Exclosure studies of garlic mustard have become common over the past decade and a half across the plant’s naturalized range. In 2018, we initiated one in the oak forest at Ordway.
Our results so far align with most (but not all) such studies: Removing deer harms garlic mustard, too. At Ordway, fewer garlic mustard plants grow within exclosure plots than outside them, and fewer adult plants are represented among the tallest plants in the plots. After two years of exclosure, garlic mustard adults represented nearly 50 percent of the tallest plants in our plots outside the fences, but less than 10 percent inside.
However, removal of deer may not be enough to shift the balance back toward native species. One recent study found that exclosure alone did not affect garlic mustard abundance, and only had modest effects on native plants. Maximum native species abundance was only achieved with the additional step of sowing their seeds. This result indicates that the ability of some native species to disperse into available space is limited, and highlights another of garlic mustard’s trait differences that could contribute to invasiveness: its dispersal strategy.
A basic problem all plants need to solve is how to spread themselves or their offspring into new habitable areas, given that they are unable to move. A staggering array of plant dispersal strategies have evolved to solve this problem. A single best strategy does not appear to exist; rather, the costs and benefits of various strategies differ across environmental conditions. The dispersal strategy used by garlic mustard in forests like the one at Ordway appears to be different from that of the native plants and, again, may give it an edge in capitalizing on recent human-induced changes.
Garlic mustard produces a lot of seeds—several times the number produced by its native neighbors of similar size at Ordway. If you jostle a garlic mustard plant that has gone to seed, the seedpods burst open, flinging their contents up to two meters away. You can hear the seeds pour down like tiny rainstorms to the ground below. Garlic mustard’s high seed output is a result of its fixed lifespan, in which the plant reproduces only once in its lifetime, right at the end. Reproduction by species with fixed life spans is often called big bang reproduction, since these species will produce as many offspring as possible in their single reproductive effort.
J. M. Heberling et al., 2019.
The benefits of high seed production to garlic mustard’s invasive ability have been demonstrated in a variety of studies. At Ordway, we have observed garlic mustard spread into new tree fall gaps much more quickly than native species. It is intuitive that the rapid spread of garlic mustard would be a result of its high seed output. However, this result is far from a foregone conclusion. Making large amounts of seeds is often not the best way for a plant to spread. Seeds take time to germinate and often need specific conditions under which to do so. Garlic mustard seeds, for example, require the drastic temperature changes of a winter–spring cycle to break dormancy, and germinate much better on bare soil than on leaf litter. New sprouts also need time to grow large enough to support themselves, during which time they are vulnerable to all manner of ill ends.
To compensate for such seed-related shortcomings, many plants use a different strategy to spread: vegetative reproduction. The basic idea here is to spread by growing wider. A common approach used by the native woodland herbs, including several species at Ordway, is to spread via horizontal roots or stems that sprout new stalks at intervals along their growing length. This strategy allows parent plants to spread offspring into new habitats right away, to spread into areas where seed germination might not be successful, and to support new sprouts via physical connections through the most vulnerable portion of their development. Compared with seed dispersal, however, vegetative spread is limited in terms of the distance from the parent that offspring can explore. Likely for this reason, plant species using it generally also produce seeds, often equipped with specialized features to exploit wind or animals for longer-distance dispersal. Because such plants must allocate limited resources across two strategies, they usually produce fewer seeds than big bang reproducers such as garlic mustard.
An increasing number of researchers view garlic mustard as a passenger of changes that are already happening.
The balance between the net advantages of seed-only and mixed reproductive strategies is probably mediated by many environmental factors. An especially likely factor is disturbance, a broad term used by ecologists to indicate removal of living biomass or disruption of the physical elements of an ecosystem. Removal of biomass and exposure of bare soil by sources of disturbance can increase the advantages of seeds relative to vegetative spread. In recent decades, the deciduous forests of Minnesota—the ecosystem type most frequently subject to invasion by garlic mustard—have been exposed to an increasing frequency and variety of disturbances. Expanding human populations have removed large tracts of forest and dissected many of the remaining tracts with roads, trails, and railways. The expanding white-tailed deer populations mentioned above are denuding native forest herbs and shrubs and exposing soil with their tracks and their habit of foraging beneath leaf litter. Outbreaks of disease such as Dutch elm and oak wilt, and parasites such as the emerald ash borer, are likely increasing rates of tree loss from canopy and subcanopy layers. And earthworms, introduced by European colonists to formerly glacier-scoured areas of the Midwest, are rapidly removing the deep litter and organic layers that historically blanketed the soils of the forest floor.
It seems likely that these disturbances have collectively tipped the balance toward favoring seed-based dispersal in forest understories. For a prolific seed-producer such as garlic mustard, that could amount to a significant boon.
The emerging picture of garlic mustard invasion as a complex result of multiple changes occurring in Midwestern forests is essentially the opposite of the causal scenario that seemed evident when the plant began to be studied intensively as an invader. Early on, it seemed likely the plant was spreading by something like brute force and driving undesirable changes in forest ecosystems in the process.
Figures courtesy of the author.
Today, an increasing number of researchers view the plant mostly as a passenger of changes that are already happening. A deeper look through observation and experiment has revealed many factors that push back against the plant’s most antagonistic traits, and has identified a suite of other likely reasons for its spread. If native ecosystems in garlic mustard’s invaded range are sick, the plant is beginning to seem more like another symptom rather than the disease. Strikingly, many of the same people who initially sounded the alarm about garlic mustard have become the most strident champions of this updated view. Their public reversal is a textbook case of science working the way it is supposed to.
The willingness to update hypotheses in light of the best current evidence is one of the features that sets science apart as a uniquely reliable method of inquiry. In the case of invasion science, the quality of information generated by this approach holds many practical benefits in addition to theoretical ones. Understanding the actual causal relationships that underlie the correlation between garlic mustard spread and diminishing local diversity of native plants can help land managers avoid wasting limited resources on interventions that may be ineffective or even harmful.
Scientific understanding is far from perfect, of course. The updated view of garlic mustard comes with many caveats. The balance of factors aiding garlic mustard undoubtedly differs among sites, and competition or allelopathy may be important in some. It is also possible that garlic mustard will turn out to be a “backseat driver” that relies on environmental change to invade, but causes further harm to ecosystems once it arrives. This possibility is being studied by several groups, including ours. Whatever understanding we develop for garlic mustard will not directly apply to any other invasive species. Understanding causes and consequences is most reliable on a species-by-species basis. In the case of garlic mustard, for better or for worse, we may be the ones in the driver’s seat.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.