
This Article From Issue
January-February 1998
Volume 86, Number 1
DOI: 10.1511/1998.17.0
In the fall of 1987, Steven Chu had just taken a position as professor of physics at Stanford University. Writer Keith Wailoo (now a professor in the Departments of Social Medicine and History at the University of North Carolina at Chapel Hill) dropped by Chu's offices at Bell Laboratories to interview the young scientist, who had developed an optical technique for slowing down atoms so that they could be studied—work that has led to many applications and was antecedent to the recent creation of a new state of matter called the Bose-Einstein condensate. Atoms in a gas at room temperature zip around at about 4,000 kilometers per hour, but Chu uses the light from lasers to "cool" gases to temperatures in the microkelvin range, which slows the atoms to speeds of a few tens of centimeters per second. Chu says that laser light acts as a sort of optical molasses. Associate Editor Mike May recently interviewed Chu again, after he was selected, along with Claude Cohen-Tannoudji and William Phillips, to receive the 1997 Nobel Prize in Physics. We begin with a few excerpts from the interview published in our January–February 1988 issue. The sketches are from Steven Chu's notebooks.

Stanford University News Service
1988
Could you explain something about the physics of slowing those atoms down—of creating what you came to call optical molasses?
It's very simple. When an atom absorbs a photon from the laser, it absorbs the momentum of the photon. And every time it absorbs a photon, its velocity slows down by three centimeters a second. It then reradiates the photon with no preferred direction; what this means is that if you average over many photon absorptions, the atom constantly gets momentum kicks in the direction of the laser beam.... [T]he atom can absorb a photon and reradiate it in about 30 nanoseconds. So in the course of one second you can get a pretty substantial force on the atom, something like 100,000 times the force of gravity. This means that over the course of a millisecond you can slow an atom to an insect's crawl.
What are the actual dynamics of the molasses?
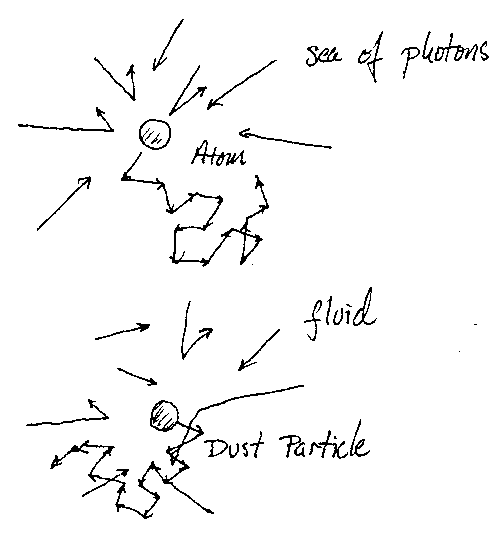
When I was making a back-of-the-envelope calculation of how long the molasses would keep the atoms in this region, I was thinking of a way to simulate this by computer, and I thought, "Well, you really don't need a computer simulation, because you can make an analogy to Brownian motion." The situation is very similar to Brownian motion where you have a dust particle and you put it in a fluid. As the atoms hit against this dust particle it starts to jostle around, and the fluctuations in the number of atoms that hit from the left and the right actually make the dust particle move. But the fluid dampens its motion; once it starts to move it hits viscosity in the fluid and wants to slow down. It's exactly the same in optical molasses, only it's a fluid of photons from the laser that creates the dampening. If the atoms want to move in the fluid they slow down, and it looks exactly like Brownian motion.
So after scratching around for a half hour or so I said, "Hey! I know about this, I learned this in elementary physics!" In fact, Einstein was the first guy to figure out Brownian motion; it's a lesson that everybody learns. Doing the calculation, this random-walk motion meant that if you had a region about a centimeter in diameter you could keep the atoms corralled for about a second.... [I]f you arrange laser beams in a certain way, amazing things should happen; namely, you should create a soup of photons that would damp any motion of the atoms, so if the atom wants to go any particular way it can't, it just sits there.
Intuitively, what did you expect to happen? Did you expect some other result?
We were expecting that the damping forces that slowed down the atom would not be that strong ... [but we] put the atoms in the optical molasses, and they cooled to the temperatures predicted and they hung around for the time predicted.
Steven Chu
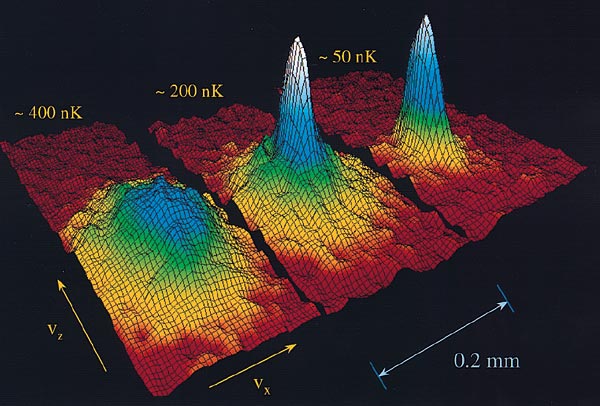
Of course, in optical molasses the atoms eventually diffuse away, whereas the ultimate goal is to trap high densities of atoms indefinitely. So optical molasses doesn't have quite the same mystique as the optical atom trap, which was supposed to do these things. The optical trap was one of those brass rings that people wanted.... [T]here are a lot of exciting things that scientists around the world are hoping to do with it. In particular, we could look at what we call the quantum statistics of these atoms. Will they obey Maxwell-Boltzmann statistics the way classical atoms do, or will they obey Fermi-Dirac statistics or Bose-Einstein statistics? The difference is immense.... In Bose-Einstein statistics [atoms] actually like to be in the same quantum state, so if you start piling them one on top of another and get them cold enough, what's predicted is that the atoms will begin to form a single macroscopic quantum state, a Bose-Einstein condensate. We are now at a temperature of about 300 microkelvin, and if we can go down by another two orders of magnitude, to a few micokelvin, I think there's a reasonable probability that we will see the Bose-Einstein condensation.
1998
When Eric Cornell, Carl Wieman and their coworkers at the National Institute of Standards and the University of Colorado created the first Bose-Einstein condensation of a dilute gas in 1995, what was your reaction to that accomplishment?
I thought they did a superb job. It was wonderful. There were a number of groups that were chasing after that. In fact, I had a small effort—going about it in a very different way. It wasn't clear at the time who was really going to win this contest. Wolfgang Ketterle was certainly in the running, as well as Carl Wieman and Eric Cornell. In fact, Wolfgang also got it a few months later.
What would you say is the significance of creating this condensate?
So far, there hasn't been either a dynamite application or a change in the way we think about physics. If you consider sort of landmark physics, it's usually one of those two. You invent a new technique, or develop a new technique or new instrument or new something, like the laser, like nuclear magnetic resonance or the scanning tunneling microscope or even laser cooling. Those are all techniques. They're based on quantum mechanics, we understand those things, we apply them in novel ways, and those things have a wide impact on science because they are general-purpose techniques that get used in many many ways. There are other things—like the discovery of the tau lepton, the unification of weak and electromagnetic interactions, parity nonconservation or the invention of quantum mechanics—which really change the way we think about physics. Those are what one would think of as the very glamorous things; but on the other hand, if you think about the invention of the telescope, the microscope, the laser, and how much cumulative effect those instruments have had on the progress and the path of science—not only physics but all of science—you have to say that they were certainly of equal importance. Now, let's come back to Bose condensation. I personally think that there is a very good chance that either something new might be discovered or, at the very least, that it could lead to a new way of preparing atoms to do other experiments, just as laser cooling and trapping was used to get Bose condensation.
What have been some of the other applications of atom cooling and trapping?
There have been many applications. Mark Kesevich has exceeded the sensitivity of the most sensitive commercially available laser gyroscope with an atom interferometer. In my lab, we've surpassed the sensitivity of the best absolute-measurement gravimeter, which measures the acceleration due to gravity. So you think that's kind of crazy, that by dropping atoms you can make the most sensitive measurement of gravity, but it's true. Also, in a very very short period of time (a year and a half), one of my postdocs, Kurt Gibbles (now a professor at Yale University), constructed an apparatus that exceeded the short-term stability of the atomic clock maintained by the National Bureau of Standards. These are applications we've developed at Stanford that have already exceeded technologies that were developed and refined over the past 30 years.
Steven Chu
Beginning around 1989–1990, I started trying to develop a technique to manipulate biological molecules. Using molecular-biology techniques, we glued little polystyrene spheres to the ends of DNA molecules. Those little spheres act as handles, and using this optical-tweezers trap—which we first demonstrated in 1986 at the very beginning of all this stuff—we could hold onto DNA molecules and stretch them out and do all sorts of other stuff. So, we were doing polymer experiments, using DNA as a model polymer. Those experiments had a humble beginning: We were verifying longstanding conjectures that formed the basis of a lot of polymer dynamics but hadn't been completely verified before. Most recently, we're finding out that there are certain classes of problems where the old ideas simply were not appropriate. In fact, it was only by studying the behavior of many individual molecules that we found that any mean-field theory—that is to say, a theory based on the average behavior of the molecules—would be inadequate, and there are certain regimes where equilibrium statistical mechanics would also not apply. We can now see a very clean breakdown of these so-called "mean-field" theories: The unraveling of a polymer cannot be described by an "averaged" theory. This is beginning to have an impact on the polymer community, and it involves the same type of optical trap that I used on atoms in 1986.
Do you see other fundamental questions that might be answered by using an optical trap?
Yes. Carl Wieman is pursuing one of them, as are other groups, and it's the trapping of radioactive isotopes using the so-called magneto-optic trap that we demonstrated at Bell in 1987. The magneto-optic trap is a "people's trap" in that it is a very user-friendly device that has made cooling and trapping accessible to many many groups. It works so well that you can trap short-lived radioactive isotopes where the number of atoms is so small that conventional atom-handling techniques would not be appropriate, because there are just too few atoms. In an accelerator, for example, you can make them and whisk them away and collect them in your little light bottle. So the idea here is that with these radioactive isotopes of very heavy atoms, there are certain experiments that probe some of the high-energy questions. These would be typically parity-nonconservation experiments and experiments that look for a breakdown of time-reversal invariance. In fact, my group is starting one of the experiments to look for breakdown of time-reversal invariance using trapped atoms.
Eric Cornell
We're also trying to make a better measurement of the fine-structure constant, which sets the scale of the electromagnetic coupling between photons and charged particles, for example. Most people say, "Oh, adding another decimal place to a well-known number." Phrases like "Rococo science" come to mind—where you're just gilding the last of the lilies—but that's not quite true. The fine-structure constant has been measured in many ways, because of its central nature in physics. It's been measured in high-energy experiments. It's been measured in exotic atoms. It's been measured using neutron interferometry. It's been measured in condensed-matter experiments. In my lab, we are measuring how much an atom recoils, how much its velocity changes when it absorbs a single photon, and if we can measure that accurately—by accurately, we mean a part per billion, or a few parts per billion—then that would give us the most accurate measure of the fine-structure constant compared to any technique. Once we improve the measurement, we can re-interpret the other experiments. For example, the neutron-interferometer measurement can be used to get Avogadro's number. I am very confident that we are going to get there.
In measuring the acceleration due to gravity, our experiments now have an absolute accuracy on a scale of a few parts per billion, and we have a relative precision of a hundred parts per trillion now. How precise is 100 parts per trillion? Suppose you are on the surface of the earth. The gravitational attraction may be computed by assuming all the mass is centered at the center of the earth, 4,000 miles away. With the resolution we have—a hundred parts per trillion—if you go 300 microns further away from the earth, you will see a change in the acceleration due to gravity.
When Keith Wailoo interviewed you one decade ago, you called yourself "the slow one" in your family and said, "There's a little bit of insecurity when I'm doing physics." Has receiving the Nobel Prize changed your thoughts about that?
No. I'm not smarter. My IQ has not gone up, and probably it's gone down. Look, I'm not an Einstein. It depends what you mean by smart. If it means you can read something and, in the shortest possible time, regurgitate back what was said or understand something really complicated, I'm definitely not that. There's still an insecurity. There are two parts of the insecurity that I think I would share with many of my colleagues. One is a fear that you're going to dry up and not going to be able to come up with a new idea—a really new idea. There's always a fear that you've had your last good idea, and that continues. The other's a fear that you can't really pull off the hard experiments anymore.
To learn to "pull off experiments," what skills would you recommend that young scientists should pursue most actively?
You want to try to put something that you learn in your own language, so that it's no longer something that's merely memorized but something you transfer from your head to your gut—you simplify it and put it in your own language to the point where it seems almost obvious and intuitive. It's only when you understand your science in this very obvious, intuitive way that you have a chance of thinking of something new. Thinking of something new, at least for me, does not come from hammering out a lot of long, involved equations. Even when you have to do a calculation, there is something before that which is guiding why you are doing that calculation, and it usually comes from a very intuitive, almost primal, level.
Learning science and thinking about science or reading a paper in science is not about learning what a person did. You have to do that, but to really absorb it, you have to turn it around and cast it in a form as if you invented it yourself. You have to look and be able to see things that other people looked at and didn't see before. How do you do that? There's two ways. Either you make a new instrument, and it gives you better eyes, like Galileo's telescope. And that's a great way to do it, make such a nice instrument that you don’t have to be so smart, you just look and there it is. Or you try to internalize it is such a way that it really becomes intuitive. Working on the right problem is only part of what it takes to succeed. Perseverance is another essential ingredient.
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.