Immune to Fear
By Christopher Brodie
Lupus antibodies in mice alter memory and emotion
Lupus antibodies in mice alter memory and emotion

DOI: 10.1511/2006.58.121
When antibodies fail to distinguish between your own body and an invading microbe, they make you sick. This happens in chronic autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus, which have complex constellations of variable, often debilitating symptoms because of autoantibodies—antibodies that target the body's own tissues.
Bruce T. Volpe and Betty Diamond
Although it might make intuitive sense that autoantibodies to connective tissue afflict the joints and those against motor neurons impair movement, autoimmune diseases are seldom so tidy in their causes and effects. Impaired memory and altered mood are common to many autoimmune diseases, and even some "classic" neurologic and psychiatric conditions—movement disorders, schizophrenia, autism and others—are linked to antibodies against the brain. However, unlike joints and peripheral nerves, the brain is sequestered behind a barrier that excludes large molecules such as antibodies. Exactly how antibodies get into the brain and effect changes in behavior and function is poorly understood.
Recent reports help to fill that void. Using mice that were immunized to carry one of the principal antibodies found in lupus patients, investigators led by Betty Diamond at Columbia University Medical Center report that infection or stress can allow the antibodies to penetrate specific parts of the brain, killing neurons and changing behavior.
The authors had previously shown that most of the 1.4 million people with lupus in the United States carry antibodies against double-stranded DNA. Some of these antibodies cross-react with proteins, particularly a string of five amino acids: aspartate-tryptophan-glutamate-tyrosine-serine, or DWEYS, to use their one-letter abbreviations. The team searched the database to find proteins with that sequence and found two parts of a receptor for the neurotransmitter glutamate: the NR2A and NR2B subunits of the so-called NMDA variety of glutamate receptor.
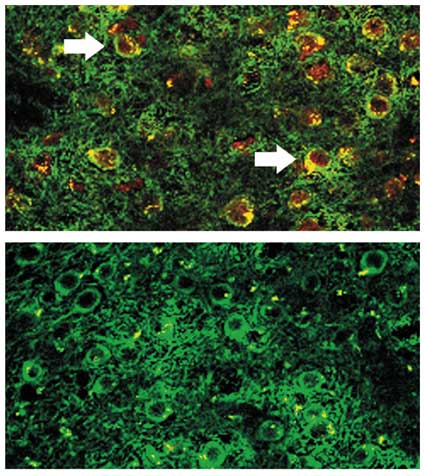
Injecting the DWEYS peptide into mice caused them to develop antibodies similar to those found in lupus patients, but the antibodies had no immediate effect on cells or behavior, presumably because they were excluded from the central nervous system by the blood-brain barrier. But this defense can be compromised, by head trauma, bacterial toxins, osmotic changes or stress in the form of a spike in adrenaline. In a recent paper (Immunity, August 2004), Diamond and her colleagues used an injection of lipopolysaccharide, a bacterial endotoxin, to breach the barrier and allow antibodies to cross into the mice's brains. The antibodies bound to neurons in the hippocampus, a structure rich with NMDA receptors, and killed a significant number of cells within a week. Not surprisingly, those mice scored worse than controls on memory tests that require the hippocampus; performance on non-hippocampal tasks was normal. The lipopolysaccharide had no effect on mice that had not been immunized with the DWEYS peptide.
The scientists determined just how the cells died by giving the mice memantine (which blocks the NMDA receptor) prior to opening the blood-brain barrier: The neurons were spared, and behavior was normal. This result shows that the cause of cell death was overstimulation of the NMDA receptors. The neurons didn't die because the antibodies incurred the wrath of the immune system; they died because their uninterrupted firing was toxic.
The follow-up paper was published online January 4 in Proceedings of the National Academy of Sciences. The study design was similar, except that the authors used an injection of epinephrine (adrenaline) rather than a mock bacterial infection to penetrate the blood-brain barrier. Surprisingly, cells in the hippocampus were spared. The mice did fine on memory tests and behaved normally. But neurons in the amygdala were half dead. This part of the brain regulates emotion—particularly fear—and is essential for a Pavlovian behavioral task called fear conditioning. In this test, mice are placed in a special cage and conditioned to associate a mild electrical shock with distinctive sensory cues such as novel smells, textures or sounds. Later, when confronted with the same cues, the animals anticipate the shock, get scared and freeze. But the mice with lupus antibodies that received the adrenaline could not muster the same response of fear. As before, memantine prevented the anatomical and behavioral changes. Furthermore, treatment with a version of the DWEYS peptide had a similar protective effect, presumably because it bound the antibodies before they reached the NMDA receptor.
This story would be interesting enough by itself—preclinical studies of a lupus drug based on the peptide are under way—but the implications for other diseases are even bigger. Viruses, bacteria, parasites and vaccines have all been implicated in neuropsychiatric conditions, including schizophrenia and autism. But scientists seldom know how or why an infection leads to behavioral consequences. Clearly it is more than infection alone: Not every person with toxoplasmosis develops schizophrenia, and not every schizophrenic has antibodies to Toxoplasma gondii. Diamond's results offer one example of how to connect these dots.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.