Going Against the Flow
By Fenella Saunders
Sometimes particles prefer to propel themselves uphill
Sometimes particles prefer to propel themselves uphill

DOI: 10.1511/2006.60.313
Particles strive for the life of a couch potato—sinking into a spot that has the least energy, where gravity can't pull them down any farther and movement is at a minimum. Getting a particle moving requires keeping it off kilter, out of equilibrium. But particles in such a state tend to bounce all over; harnessing their movement in a single desired direction is the goal of many nanoscale devices.
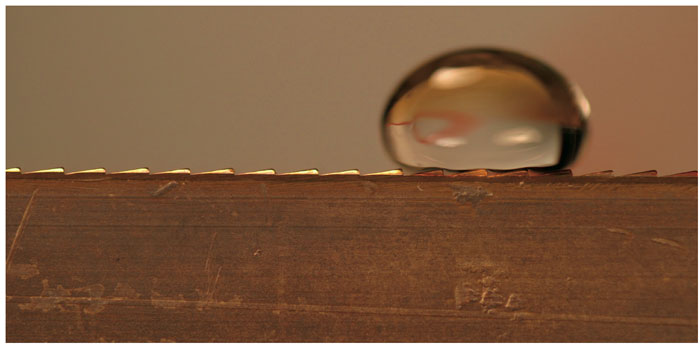
Photograph courtesy of Benjamin Aleman and Michael Taormina, University of Oregon
One way to do this is with a ratchet effect—a mechanism that uses spatial asymmetry and energy gradients to make movement easier in one direction than another. It turns out that in some cases, ratchets not only control movement, but can also move particles in unexpected directions—away from a minimum energy state, the molecular equivalent of a creek climbing uphill under its own power.
Indeed, physicist Heiner Linke and his colleagues at the University of Oregon, along with collaborators at Oregon State University and the University of New South Wales in Australia, have used a ratchet mechanism to make water droplets propel themselves up an incline. Linke and his colleagues employed what's called the Leidenfrost effect, which many people have seen in their own kitchens.
If a skillet is heated to an extremely hot temperature, between 200 and 300 degrees Celsius, drops of water flicked into the pan will skitter across the surface, remaining intact for a minute or so. A surface not quite so hot will boil away the water droplets instantly, but the superheated surface instead instantly turns the bottom of the droplets into a layer of steam. Vapor is a poor heat conductor, so the steam insulates the drops from further boiling. It also provides them with a means of movement: The water drops bounce around like hovercraft on a cushion of air.
Linke and his colleagues did not use a smooth metal surface, but one covered with a sawtooth pattern. The teeth inclined more steeply in one direction than the other—an asymmetrical surface, and therefore a ratchet mechanism. Millimeter-sized water droplets piped onto the superheated sawtooth surface zip off in one direction like airport passengers on a moving walkway, reaching speeds of up to 5 centimeters per second, even if the surface is tilted so that the droplets have to climb uphill. As the investigators reported in the April 21 issue of Physical Review Letters, the phenomenon works for many other liquids, such as ethanol and liquid nitrogen, although the temperature at which the Leidenfrost effect kicks in varies from 50 to 150 degrees above the boiling temperature of the liquid.
Linke believes that the sawtooth shape of the surface ultimately gives the droplets their staunchly preferred direction. "On a smooth surface, the steam escapes evenly in all directions, so the droplets glide around randomly," he says. "We think the asymmetry of the surface gives direction to the steam." He used particles of glitter to image the puff of steam, which can be seen to exit preferentially from the advancing side of the droplet. Linke believes that this vapor release pulls along the droplet by viscous forces, much like a boat floating with the current of a river.
Such a flow might be useful in cooling systems for electronics: Waste heat from a microprocessor could power the pumping mechanism without any moving parts. The vapor layer prevents the liquid in a ratchet pump from being a good heat sink, but the ratchet could be used to pump liquid through a heat exchanger.
Linke is generally more interested in ratchets than in fluid flows. A few years ago he and his colleagues fabricated a ratchet mechanism out of a series of triangle-shaped semiconductor wells to control the random motion of electrons. The triangular well forced electrons into its pointy end and thus created an electrical gradient, making a current travel through the ratchet more easily in one direction than the other.
Another research group, however, reporting in the March 30 issue of Nature, has found that in some cases charged particles prefer to move in the "hard" direction created by a ratchet mechanism.
Victor Moshchalkov and his fellow physicists at the Catholic University of Leuven in Belgium have found that the number of charged particles in a ratchet mechanism can be a significant factor in how the ratchet works, particularly if the particles repel one another. With an odd number of particles, the ratchet worked as expected, but with an even number of particles, flow was in the opposite direction.
Moshchalkov's group studied fluxons, tiny vortices of magnetic flux that repel one another, often found in sensitive superconducting measurement devices. The fluxons were trapped in a ratchet potential made of two nanofabricated wells, one larger than the other, forming an asymmetrical shape that is essential for ratchets.
A fluxon put into the ratchet will fall into the deeper well, but if an oscillating force is turned on, the fluxon will find it "easier" to pop into the shallower well and then flow over the top of the step into the next pair of wells, providing movement of fluxons in only one direction.
However, if two fluxons are put in the ratchet, one will stay in the shallower well while the other falls into the deeper well. This time, the external force makes the fluxon in the shallower well push into the deeper well, repelling the fluxon that's already there and popping it out of the step in what is supposed to be the "hard" direction of the ratchet.
"If you have more than one particle locked into an asymmetric potential, due to the repelling interaction of particles there is a sort of mini-billiard effect between the particles," says Moshchalkov. "Then you have some strange knockout effects, and particles move opposite to the prescribed direction."
Biological membranes also act as a kind of ratchet, allowing the flow of ions in only one direction, in order to control such vital functions as heart beat and nerve firing. Moshchalkov speculates that his group's study might explain why some medications, in improper dosages, can cause poisoning. Overloading the medication may cause a membrane's ratcheting mechanism to reverse, pumping ions in the wrong direction.
"The lesson is that you have to be careful with interacting particles," says Moshchalkov. "We hope our work may be able to help specify concentration limits."
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.