A Shortcut to the Brain
By Katie L. Burke
Two studies show how cancer cells and immune cells use secret passageways through the skull marrow to the protective layer around the brain.
Two studies show how cancer cells and immune cells use secret passageways through the skull marrow to the protective layer around the brain.

Dorothy Sipkins of Duke University School of Medicine and Fanny Herisson of Harvard Medical School had independently begun asking similar questions during clinical medical work early in their careers. Sipkins, who had been studying cancers that metastasize to the central nervous system (CNS), wondered how cancer cells get into the brain. Herisson wondered whether and how the skull’s marrow contributes to brain inflammation. Both researchers honed in on blood vessels that had not previously been scrutinized—tiny ones that pass through the skull’s marrow to the outer covering of the brain, the meninges.
“In anatomy class in med school, there’s so much to learn,” Sipkins explains. “We physicians need to know lots of important vessels—but not all of them.” These emissary vessels were obscure but not completely unknown in anatomy, because they can be a gateway for bacteria to cause meningitis in head injuries. It took Sipkins years of puzzlement before she saw the clues that led her to look in the right place.
Fanny Herisson, M.D., Center for Systems Biology, Massachusetts General Hospital
Herisson also didn’t start out looking for these channels. “As a clinician, I had no idea they existed,” she says. “We think of the bone and brain as two different compartments, and the meninges are between them. I had no idea that there might be some kind of communication between them.” But she thought there could be. “The skull and its marrow cavity are right next to the brain,” she says. “And yet nobody had ever looked into potential interactions between the two compartments.”
Both teams have now identified the channels’ role in molecular communications. Sipkins’ team found a receptor on particular cancer cells that guides them to the CNS—and could be a treatment target. Herisson and her colleagues discovered that these channels have an immune function as well. Sipkins’ and Herisson’s studies, published respectively in Nature in July and Nature Neuroscience in August, open up ways to study and potentially treat brain diseases, including cancers, multiple sclerosis, and Alzheimer’s disease.
More than 10 years ago, when Sipkins was a fellow at the Dana-Farber Cancer Institute, she worked with patients who had acute lymphoblastic leukemia (ALL), a blood cancer that is especially prone to metastasizing to the brain and spine—so much so that all patients with ALL are preventatively treated with chemotherapy in the spinal fluid.
Sipkins started looking through a microscope at blood vessels of mice with ALL, in a layer of the meninges where the cancer cells are found early. “We looked and looked, but we never saw leukemic cells in our mouse models that were in circulation going through those superficial vessels,” Sipkins says. “We were really puzzled and put it aside for a long time.”
When her lab moved to Duke in 2014, she began collaborating with Gilead Sciences. They make a drug called idelalisib, which is already used to treat leukemias and lymphomas. Idelalisib inhibits an enzyme in a signaling pathway that can go awry and be involved in cancer.
“We gave idelalisib to the mice [with ALL], and they lived longer,” Sipkins explains. “But they had the same amount of disease in the rest of their body. They just weren’t developing CNS disease.” At first, she and her collaborators thought that the drug must be more effective at killing cancer cells in the brain. But the drug’s levels weren’t higher in the brain. That meant that the drug must prevent cancer cells from getting into the CNS.
“We did a screen, which is what you do when you don’t know what the heck is going on,” Sipkins says. They isolated leukemic cells from mice that were treated with idelalisib, and looked for which genes were turned on or off more often than in untreated mice. They noticed an interesting molecule, called α6 integrin, which is part of a cell receptor that binds to another molecule called laminin, which is part of the extracellular matrix, the network of molecules that provides structure and support for cells.
They found that α6 integrin was expressed in the majority of ALL cases. “When we looked at where laminin is found in the CNS, it’s all in the meninges, wrapping specific blood vessels,” Sipkins says. When she looked at studies of α6 integrin, she learned that mice without this molecule have abnormalities in neural development. “So, α6 integrin is important for neural cells to find their way in the developing brain and helps them identify where they are with respect to the meninges,” she says. These molecules were in the right spots. Why had they missed them?
“I thought, ‘I’ve got to review my anatomy, because clearly I’m missing something,’” Sipkins says. “I was looking at the anatomy of the skull. I saw this image of blood vessels that go from the bone marrow through these apertures in the bone, down into the meninges, and then become meningeal vasculature on the other side. That was the moment when I was like, ‘Oh my God.’ Who knew?”
Then they looked microscopically at the emissary vessels in the spines of diseased mice. “In the leukemic mice you see these vessels surrounded by leukemic cells,” Sipkins says. “They were in these tunnels, surrounding these blood vessels, migrating through.” The cancer cells had found a shortcut secret passageway into the brain and spinal cord—one that they had special keys to. Now that those keys are known, Sipkins hopes they can be used to target treatments for ALL and other brain cancers.
Herisson says, “I was happy to see Sipkins’ paper, because it shows in another mouse model a disease context where these channels are relevant. Her research shows a different mechanism for the cells to travel through the channels [than my team found], but that’s still another example of communication between compartments.”
Herisson’s research describes an immune function for the same channels. They provide a way for more first-responder white blood cells to get to acute brain injuries than those from marrow in a leg bone. This result contests the current thinking that after an injury the marrow homogeneously releases white blood cells into circulation to be recruited indiscriminately to the site of inflammation.
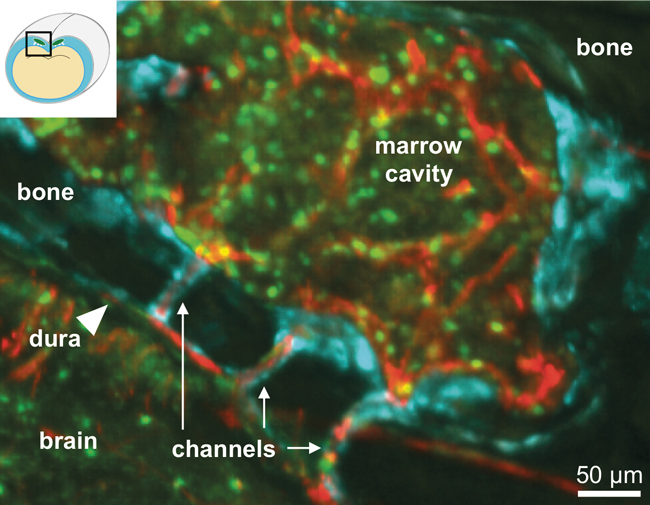
To show the contributions of white blood cells from bone marrow in different locations, Herisson and her colleagues developed a novel method. “We came up with a technique to look specifically at what the tibial marrow would do and what the skull marrow would do,” she says. “It’s kind of like a tattooing technique.” In mice with stroke or meningitis, which cause a big inflammatory response, Herisson and her team injected a fluorescent green tracker, which attached to white blood cells of interest in the marrow in the leg, and a fluorescent red tracker in the skull marrow. The red cells from the skull marrow showed up in the meninges in greater numbers than those from the leg bone.
Herisson then looked at how the immune cells were traveling from the skull to the meninges through the emissary vessels, both in vitro and in vivo in mice. “Looking in vivo was very challenging,” she says. “The blood flow goes from the dura [a layer of the meninges] outside to the skull, and the cells go in the opposite direction. You can see the cells crawling as they go against the flow.”
Herisson thinks this inflammatory process could be important in many neurological diseases, including autoimmune diseases such as multiple sclerosis. “Mouse models for multiple sclerosis show meningeal inflammation before the brain or spinal cord gets inflamed,” she says.
The work by both Herisson and Sipkins shows how cells can efficiently get to the meninges. The cells do not cross the blood–brain barrier, unless the meninges are damaged. These studies open up many questions about whether other molecules use these channels, and how they affect disease and immune responses.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.