Getting a Fix on Pollen Folding
By Anna Lena Phillips
A collaborative study illuminates old questions about how pollen grains survive
A collaborative study illuminates old questions about how pollen grains survive

DOI: 10.1511/2010.85.292
When a flowering plant releases pollen from its anther, the tiny grains rapidly desiccate, their outer layers folding to keep the genetic material inside safe until they reach the stigma of another flower. The process was given a name, harmomegathy, by palynologist Roger P. Wodehouse (1889–1978). But how it works had never been investigated. “The problem was not known to physicists, and the mathematical technique was not known to biologists,” says Eleni Katifori, a postdoctoral physicist at the Rockefeller University’s Center for Studies in Physics and Biology. During her Ph.D. work at Harvard, Katifori and her collaborators used computer simulations and scanning-electron microscopy of pollen grains to try to determine the geometric pathways by which they fold. The results are published in the April 27 issue of Proceedings of the National Academy of Sciences.
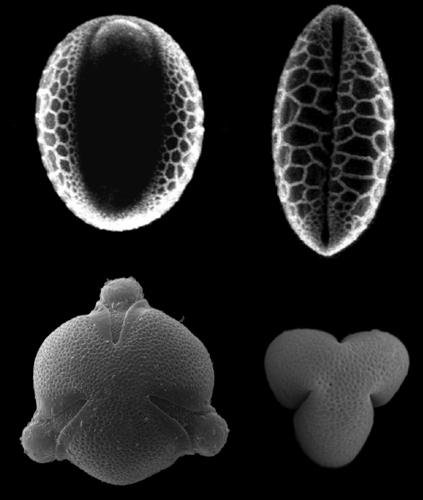
The team chose pollen from Lilium longiflorum and Euphorbia milii, whose structures are common among angiosperms. The lily pollen has one elongated aperture through which sperm cells exit; the euphorbia has three long colpi, or furrows. The group simulated the pollen wall with thin-shell structures, for which stretching requires more energy than bending. The models closely matched what happens in the actual pollen. “This is the unifying aspect of the mechanism,” says Katifori. “You have a big change in shape but with no stretching, just bending. Like origami—just by bending a flat piece of paper, you get very weird things.”
In the case of the lily pollen, what you get is something like a football—and protection for the delicate material within as it hurtles through the air. Imagine a hollow sphere with a slice missing—this represents the aperture—and then press at either side of the slice so that the edges meet. “Like it’s a clown’s nose,” says Katifori. Simple enough. The E. milii, with its three colpi and complex curvature, takes a little more work. The sphere becomes wider and flatter and the colpi crumple inward, allowing the pollen walls to meet on either side of them.
Nan Crystal Arens, a palynologist at Hobart and William Smith Colleges, wonders how the design of harmomegathy developed during plants’ transition from water to land. Studying early land plants using this quantitative approach could “allow us to test some assumptions that we have taken on logical faith.” Placing structure in evolutionary context is just the kind of insight that future interdisciplinary work could engender. “There are several areas of biology where such collaboration would be very, very fruitful,” says Patricia G. Gensel, a paleobotanist at the University of North Carolina at Chapel Hill. It’s easy to think that one’s own field would not have much to say to a vastly different one, she says, but “when you get a conversation going, you realize, there are ways this could be useful to me.”
For Gensel, the research also raises questions about the other end of evolution. She notes that the two pollen forms studied are common to gymnosperms and older groups of angiosperms—so “the direction of evolutionary change appears to be away from their design.” More work on folding in advanced angiosperms is in order, Gensel thinks, “because there apparently is more than this one way to close down when drying out.” Likewise, it seems clear, there are many more ways to explore complex questions at the intersection of physics and biology.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.