Designing for Recycling
By Katherine Harry, Emma Rettner
A new process for creating polymers results in plastics with customizable characteristics and reuse baked in.
A new process for creating polymers results in plastics with customizable characteristics and reuse baked in.

DOI: 10.1511/2024.112.1.6
Hundreds of millions of tons of single-use plastic end up in landfills every year, and even the small percentage of plastics that get recycled can’t stay out of the trash forever. But our group of materials scientists has developed a new method for creating and deconstructing polymers that could lead to more easily recycled plastics—without requiring consumers to carefully sort their recycling on trash day.
Synthetic plastics were first developed in the early 20th century, and their use became widespread after World War II. In the years since, people have come to understand the enormous impacts—beneficial as well as detrimental—plastics have on human lives and the environment. As polymer scientists dedicated to inventing sustainable solutions for real-world problems, we set out to tackle the issue of plastic waste by rethinking the way polymers are designed so we could make plastics with recyclability built right in.
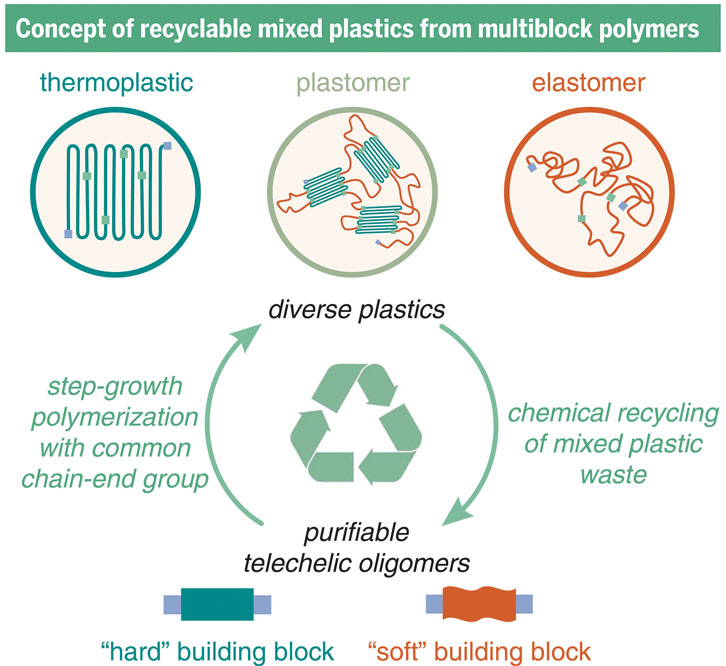
From Zhao, 2023.
Everyday items including milk jugs, grocery bags, takeout containers, and even ropes are made from a class of polymers called polyolefins, which make up around half of the plastics produced and disposed of every year.
These polymers are used in plastics commonly labeled as #2 (high-density polyethylene, used in opaque containers such as milk jugs), #4 (linear low-density polyethylene, used in squeezable bottles and grocery bags), or #5 (polypropylene, used in straws and microwave-safe containers). These plastics are incredibly durable because the chemical bonds in those polymers are extremely stable. In a world set up for single-use consumption, durability is no longer a design feature but rather a design flaw.
Imagine if half the plastics used today were recyclable through twice as many processes as they are now. According to the Organisation for Economic Co-operation and Development’s 2022 publication Global Plastics Outlook, only 9 percent of plastic waste is currently recycled. Doubling the tons of plastics recycled would make a dent in the plastics accumulated in the environment.
Even the plastics that make it to a recycling facility can’t be reused in exactly the same way they were before, because the recycling process degrades the material, resulting in lost utility and value. Instead of making a plastic cup that is downgraded each time it gets recycled, manufacturers could potentially make plastics once, collect them, and reuse them again and again.
Conventional recycling requires careful sorting of all the collected materials, which can be challenging with so many different plastics. In the United States, collection happens mainly through single-stream recycling, in which metal cans, glass bottles, cardboard boxes, and plastic cups all end up in the same bin. Separating paper from metal doesn’t require complex technology, but sorting a polypropylene container from a polyethylene milk jug is difficult to do without the occasional mistake. And when two different plastics are mixed together during recycling, their useful properties are greatly reduced—to the point of making them useless.
But say you could recycle one of these plastics by a different method, so it didn’t end up contaminating the recycling stream. When we mixed samples of polypropylene with a polymer we made, we were still able to depolymerize—or break down—the material and regain our building blocks without chemically affecting the polypropylene. This success indicated that a contaminated waste stream could still recover its value, and the material in it could go on to be recycled, either mechanically or chemically.
In a study published in Science in October 2023, we described a series of polymers with only two simple building blocks—one soft polymer and one hard polymer—that behave like polyolefins but could be chemically recycled.

WorldPix/Alamy Stock Photo
Connecting two different polymers together multiple times until they form a single, long molecule creates what’s called a multiblock polymer. But making these multiblock polymers is easier said than done. To link the hard and soft polymers, we adapted a technique that had previously been used only on very small molecules. This method enabled us to couple the two polymers directly, without needing to incorporate additional molecules to form this link.
By adjusting how much of each polymer type goes into the multiblock polymer, our team produced a wide range of materials with properties that spanned polyolefin types. Using the same strategy, but applied in reverse by adding hydrogen, we could disconnect the polymers back into their building blocks and easily separate them to use again. When we made new polymers out of these recycled plastics, they performed just as well as the original materials even after several iterations of chemical recycling.
Separating paper from metal doesn’t require complex technology, but sorting a polypropylene container from a polyethylene milk jug is difficult to do without the occasional mistake.
If trends remained unchanged, annual plastic use will nearly double by 2050 according to Back to Blue, an initiative of Economist Impact and the Nippon Foundation aimed at improving ocean health. Increasing the reach of plastic recycling is crucial for managing the growing quantity of plastic waste. Considering the life cycle of a product is also an important and often-overlooked step when designing new materials.
Using just two building blocks to make plastics that have a huge variety of properties would go a long way toward reducing and streamlining the number of different plastics used to make the products we need. Instead of needing one plastic to make something pliable, another for something stiff, and a third, fourth, and fifth for properties in between, we could control the behavior of plastics by just changing the amount of each building block.
We were able to create materials that mimic the properties of the plastics the world relies on, and our sights are now set on creating plastic compositions that couldn’t be made with existing methods. Although we’re still in the process of answering some big questions about these polymers, we believe this work is a step toward more sustainable plastics.
This article is adapted from a version previously published on The Conversation (theconversation.com).
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.