Genetic Strategies for Controlling Mosquito-Borne Diseases
By Fred L. Gould, Krisztian Magori, Yunxin Huang
Engineered genes that block the transmission of malaria and dengue can hitch a ride on selfish DNA and spread into wild populations
Engineered genes that block the transmission of malaria and dengue can hitch a ride on selfish DNA and spread into wild populations

DOI: 10.1511/2006.59.238
Malaria kills more than a million people each year, primarily children under the age of six. Dengue fever is less deadly, but an outbreak can debilitate millions of people and easily overwhelm doctors and hospitals in tropical cities.
To combat malaria and dengue, health agencies try to get rid of mosquitoes, which transmit both diseases. But a scarcity of resources hampers most control programs, and the insects are increasingly resistant to pesticides after decades of patchwork spraying. The disease organisms are evolving, too: The single-celled microbes that cause malaria are becoming resistant to widely used, inexpensive anti-malarial drugs such as chloroquine, which have been the first-choice treatment for malaria. (Drug therapies for dengue virus have never been available.) Many research teams are trying to develop vaccines for these diseases, but the complex biology of the two parasites—malaria is caused by four species of the protist Plasmodium, and four different viruses, or serotypes, can cause dengue—makes it difficult to predict whether vaccination will eventually confer broad immunity. And although pesticide-treated bed nets offer a promising low-tech means of preventing bites from malarial mosquitoes at night, the mosquitoes that carry dengue bite during the day.

Photograph courtesy of the Centers for Disease Control and Prevention.
To oppose these grim realities, several research teams (including our group at North Carolina State University) are now exploring a different approach to controlling the spread of mosquito-borne diseases, one that would reduce an insect's ability to transmit disease or would induce a population crash among selected disease-carrying species. How could either of these goals be achieved? By creating genetic changes in wild mosquitoes. Biologists have already extinguished other insect pests with genetic methods and in the laboratory have blocked the transmission of dengue and malaria in mosquitoes with engineered fragments of DNA. If scientists could breed some of those same genes into the wild mosquito population, the insect's bite might still be a nuisance—but it would no longer be a threat.
Setting aside concerns over the release of genetically modified mosquitoes (more on that later), there is reason to be optimistic that this strategy might work. Thanks to the tools of molecular biology, geneticists can make very specific changes in mosquito genomes. In 2002, a team led by Marcelo Jacobs-Lorena, then at Case Western Reserve University, modified Anopheles stephensi mosquitoes with genes that blocked the development of Plasmodium berghei, a relative of the malaria parasites that infect human beings. In March 2006, a team led by Ken E. Olson at Colorado State University showed that inserting a specific transgene—a piece of foreign, engineered DNA—into the genome of Aedes aegypti mosquitoes could substantially deactivate the dengue virus within hours of the female's blood meal from an infected person. Other scientists have developed genetic strategies that simply kill the mosquitoes that carry a specific engineered gene.
But having mosquitoes that don't transmit pathogens in the laboratory doesn't help people in the wider world. Even if scientists bred and released thousands of these transgenic mosquitoes, they wouldn't have much effect on public health unless the genetically altered insects competed with (and eventually replaced) the local strains.
If mosquitoes that carried anti-parasite genes were more evolutionarily fit (that is, if they left more offspring) than mosquitoes without these genes, then more and more of the wild mosquito population would become inhospitable to parasites with each generation. For example, if parasite-infected mosquitoes had shorter lives or fewer progeny, then a parasite-killing transgene might make its bearer more fit. Unfortunately, that isn't what happens: Mosquitoes susceptible to the dengue virus or Plasmodium are almost always just as fit as those without the susceptibility.
Not only do anti-parasite transgenes fail to improve fitness, they often reduce it. This penalty exists because the transgenes in many genetically modified organisms are just inserted randomly into the genome, where they often disrupt normal genes at the insertion site. And the transgene itself encodes RNA or protein that can change cell function, thereby decreasing the insect's fitness. Transgenic strains that bore these fitness costs would go extinct if released into the wild.
Despite the challenges, the process of engineering a disease-fighting, transgenic mosquito is not merely an academic exercise. The Bill and Melinda Gates Foundation recently contributed more than $35 million to the Foundation for the National Institutes of Health for the purpose of developing transgenic mosquitoes as a weapon against insect-borne diseases, and governmental agencies and other philanthropies in the United States and abroad have also funded this research.
Regardless of what the anti-pathogen transgene turns out to be—an antiviral or antiprotist gene, a lethal gene, or something else not yet developed—the project will succeed or fail based on the ability to drive that transgene into the wild population—even if it makes its bearers less fit. A practical system to meet this need is still far away, but it is possible. Using the rules of population genetics, a number of research groups are harnessing so-called selfish DNA, which spreads without regard to the overall fitness of its host, giving the illusion of turning natural selection on its head.
The idea of designing a gene that actively spreads through a pest population without conveying some fitness advantage is not new. A Soviet geneticist, Alexander S. Serebrovskii of Moscow University, and a British biologist, Frederic L. Vanderplank of the Tanganyika (Tanzania) Research Department, sowedthe intellectual seeds for this approach in the 1940s. The two men realized independently that in certain circumstances, competition between two interbreeding insect strains doesn't favor the fitter group. This dynamic involves the genetic property that scientists call underdominance, which can actually cause the strain with greater fitness to die out.
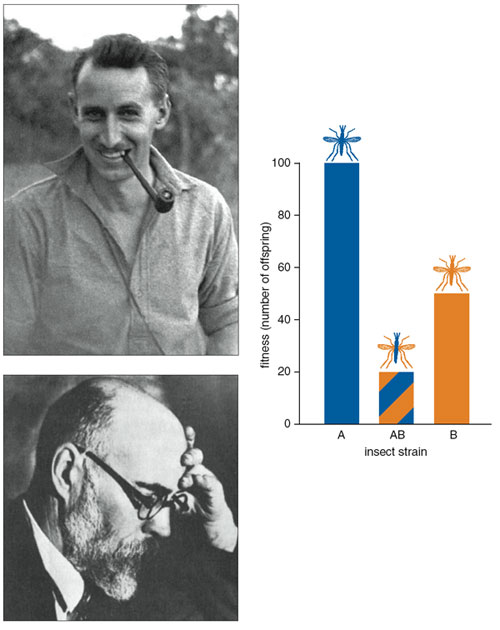
Vanderplank photograph courtesy of John Vanderplank. Serebrovskii photograph reprinted from Medvedev 1969. Chart by Barbara Aulicino.
To explain underdominance, it's helpful first to know the terms dominant and recessive, which describe the inheritance of traits. Consider a case with two purebred parents from different, interbreeding strains: Many features of their offspring will favor one parent or the other. For example, suppose that parent A comes from a strain that produces 100 eggs and parent B comes from one that produces 50 eggs. If the offspring from the mating of A and B each generate 95 eggs, a biologist would typically say that the high-egg-production trait was dominant. If, instead, the offspring laid only 55 eggs, then a biologist would classify the trait as recessive. But if offspring from the A x B mating produced fewer eggs than either parent (here, fewer than 50), then egg production would be considered underdominant. In most crosses between strains, traits do not show underdominance, but sometimes a mating between distantly related strains yields offspring that don't survive or reproduce as well as either parent. (In other words, those offspring have a lower evolutionary fitness.)
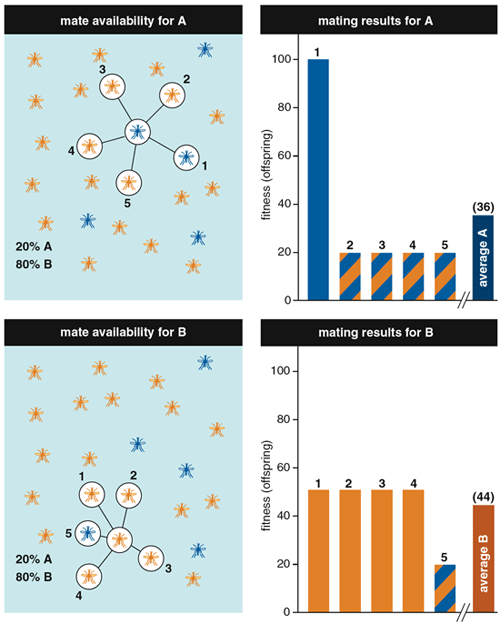
Illustration by Barbara Aulicino
Still, the idea that the less-fit strain B could outcompete the more-fit strain A doesn't seem to make sense according to basic Darwinian theory. But Vanderplank, Serebrovskii and others realized this is exactly what happens when two conditions are met: when the offspring of a mating are less fit than either parent (underdominance), and when the less-fit parental strain is more abundant. Under these conditions, adults of the less common strain A are more likely to find and mate with adults from the more common strain B, thereby producing less-fit offspring. For example, if strain A makes up 20 percent of the insects in a certain habitat and strain B makes up 80 percent, then (all else being equal) four out of five individuals from strain A will encounter a mate from strain B, but only one out of five individuals from strain B will make a match with a strain A mate. Plugging some numbers into this example: If an A x A cross results in offspring that produce 100 eggs, a B x B cross yields offspring that produce 50 eggs, and (bringing in underdominance) an A x B cross has offspring that produce 20 eggs, then the average egg production by female offspring from the A strain will be (0.80 x 20) + (0.20 x 100) = 36, and the average for strain B offspring would be (0.20 x 20) + (0.80 x 50) = 44. Even though strain A is more fit, strain B produces offspring with higher average fitness. Over time, strain B would replace strain A.
Vanderplank did exactly this experiment in the late 1940s with two sexually compatible species of tsetse flies, the insects that transmit the parasite that causes sleeping sickness. Mating the two species yielded offspring with low fitness. Working in an area that had been abandoned because of disease risk, Vanderplank released high numbers of one species into the habitat where the second was more fit. Over time, the first species outcompeted the second, sending its numbers plummeting. Within two years or so, the introduced species (which was not well adapted to the habitat) had largely died off, leaving the area free of sleeping sickness and enabling local people and cattle to inhabit the region.
Serebrovskii worked out the theory for a type of mutation called a balanced chromosomal translocation, in which a piece of one chromosome breaks off andbecomes attached to another chromosome. Serebrovskii calculated that even less-fit insects with the translocation could replace the wild-type—the normal, nonmutant strain with higher fitness—because some of the grandchildren of the cross between mutant and wild-type parents don't inherit a complete set of chromosomes (a lethal condition). Christopher F. Curtis at the London School of Hygiene & Tropical Medicine later pointed out that if the strain with the translocation carried a desirable gene (such as one that conferred malaria resistance) on the translocated chromosome, then the translocation would also sweep the desirable gene into the population. The scientists needed only to inundate the natural pest population with such a translocation.
Several research teams conducted lab and field-cage experiments in the 1970s to test Serebrovskii's and Curtis's theories. The teams made some progress with the translocation studies, but this line of investigation ultimately failed: Rearranging a mosquito's chromosomes left it unfit to survive in the field. The products of classical genetics proved too crude for the job, and interest in this approach waned.
The disinterest didn't last long. Soon after the first successful addition of foreign DNA to the fruit fly Drosophila melanogaster, biologists began to explore the potential for genetic manipulation of pest species. The growing sophistication of molecular biology has enabled them to make genetic changes with much greater precision than before. For example, Stephen Davis and his colleagues at the University of New South Wales in Australia developed a novel idea for a two-transgene system that uses underdominance to spread new genes into a population. They envisioned the creation of two distinct pieces of DNA, or constructs, that were spliced into different chromosomes. Each construct contained an on/off switch and a gene that encodes a biological toxin. The switch was "on" by default. Construct I also carried a gene that turns off the toxin production in construct II, and construct II had a gene that turns off toxin production from construct I. Thus, individuals with both constructs (or neither) survived. Having just one of them was lethal.
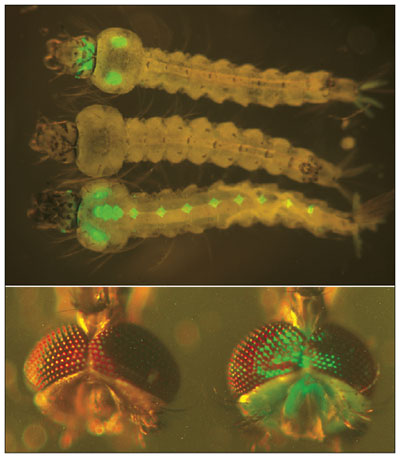
Photographs from Ito et al. 2002, courtesy of Nature Publishing Group.
In this model, a cross between a wild-type strain and a strain that was homozygous for both constructs (meaning that each construct was present on both halves of a chromosome pair) would yield progeny heterozygous for both constructs. (In other words, they would carry only one copy of construct I and one copy of construct II.) This generation would survive. But many of the second generation would die because they inherited only one of the two constructs (similar to the effect seen in Serebrovskii's translocation model).
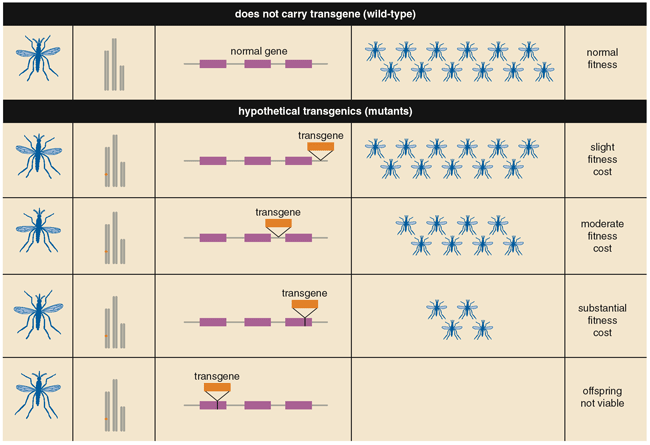
Illustration by Stephanie Freese and Barbara Aulicino
This engineered form of underdominance is superior to a translocation because it can enable the transgenes to spread even if the number of mutants released is less than 30 percent of the population (the exact proportion depends on how much of a fitness cost is associated with the transgene). Furthermore, because the engineered strains only differ from native strains by two inserted genes (instead of a full chromosome rearrangement), the transgenics should be more fit under field conditions. And as Curtis noted in the similar case of translocations, an anti-pathogen gene included in the constructs would also spread through the population. Having the anti-pathogen gene on both constructs provides a backup in the event that a random mutation disables one copy.
Theoretically, the chance of success would be even higher—and the number of engineered insects needed even lower—if the mutant strain were homozygous for two independent insertions of construct I and two independent insertions of construct II. Our research team has modeled the effects of different fitness costs and different numbers of transgenes and found that multiple insertions of a construct can be more efficient than a single insertion as long as the cost per insertion is below 10 percent. These models can predict how many lab-bred insects would be needed to eventually saturate a wild population, a number called the critical release size. It's about 15 percent when there are no fitness costs associated with multiple insertions—a tall challenge for molecular biologists. Even releasing enough engineered mosquitoes to make up 15 percent of a local population is a daunting task. When entomologists first used genetics to control pest insects in the late 1950s, they reared the insects in giant factories that could produce millions of bugs per week. These were costly operations. A transgenic mosquito program might be able to use fewer insects by releasing them during a seasonal dip in the population or after spraying pesticide to shrink the mosquito population temporarily.
Although the strategy of engineered underdominance might work in some cases, the true Holy Grail of genetic insect control would be a transgene that spreads through a population from only a few individuals. The first indication of the possibility of reaching this goal came from a transposon, or "jumping gene," a type of selfish DNA. The transposon encodes a protein that cuts the transposon DNA free of its place in a chromosome and then reinserts it in another random part of the genome. The cell's own DNA-repair machinery usually mends the hole at the original position by recreating the transposon sequence. At the end of the process, the host cell has two copies of the transposon instead of one. If this gene-hopping occurs in a cell that makes eggs or sperm, then the transposon stacks the odds that it will be passed on to future offspring. If the transposon doubles each generation (assuming for now that it doesn't harm the organism), then it will become increasingly common until all individuals in the population carry one or more copies.
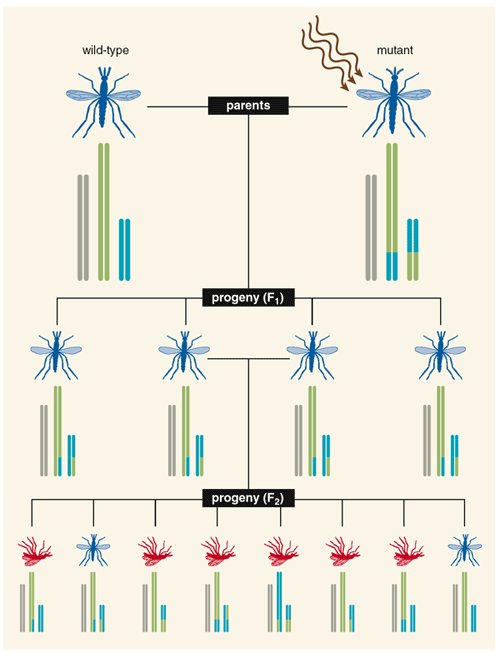
Illustration by Stephanie Freese and Barbara Aulicino
This basic scenario occurred "naturally" in Drosophila melanogaster populations throughout most of the world in the past 60 years. Today, almost any fruit fly collected from an overripe banana, whether in New York City or Nairobi, contains a transposon called the P-element. Yet the descendants of fruit flies isolated in laboratories before 1950 lack this transposon. Scientists do not know the origin of the P-element but do know that it spreads rapidly when introduced into a naive laboratory population.
Several entomologists have noted that "loading" an anti-pathogen gene into a mosquito transposon could eventually confer disease resistance to an entire mosquito population as the transposon jumped from site to site through successive generations—even if the mosquitoes that bore these genes had lower fitness. However, this is not a sure-fire strategy (use of the word "could" instead of "would" in the previous sentence was deliberate). In one experiment with D. melanogaster in the laboratory of Margaret G. Kidwell at the University of Arizona, the transposon spread as predicted through the naive flies, but the loaded marker gene was lost. The investigators concluded that at some point during the experiment, one or more copies of the transposon must have "unloaded" the added gene. The cargo-free transposons seemed to replicate faster than their loaded counterparts and eventually displaced them. Clearly, scientists will need to stabilize the genetic composition of any transposon used for practical goals.
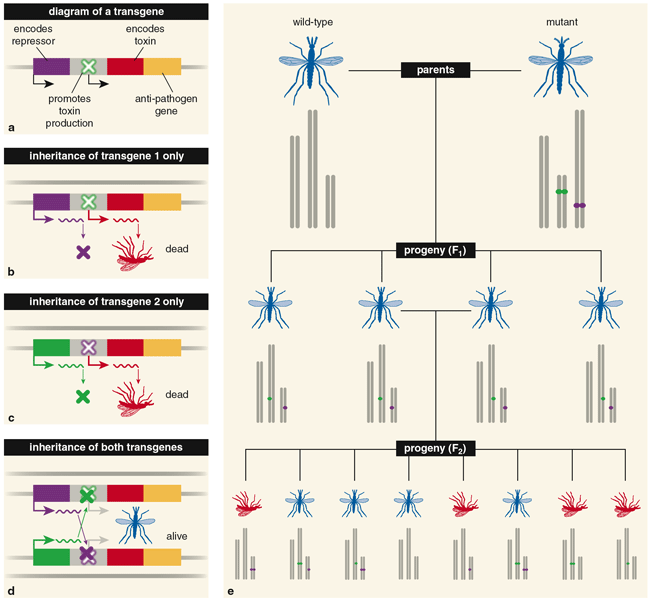
Illustration by Stephanie Freese and Barbara Aulicino
In addition to transposons, molecular biologists are studying several other types of selfish DNA that could spread a desired gene into a mosquito population. One Gates-funded project led by Austin Burt at Imperial College London focuses on a curious DNA sequence called a homing endonuclease gene (HEG). The HEG has the unique ability to copy itself from one chromosome to the identical site on the other chromosome in the pair. It accomplishes this feat by encoding a protein that recognizes the DNA sequences on either side of the HEG. When the protein, called a homing endonuclease, spots the same DNA pattern on the twin, or homologous, chromosome, it snips that sequence in two. The cell mends this double-strand break by using the HEG-containing chromosome as a template for patching the cut DNA. This repair incorporates the entire HEG sequence, and the cell becomes homozygous for the HEG. If the process of DNA cutting and repair happens in a cell that later forms sperm or eggs, then it's possible that nearly 100 percent of the offspring could inherit the HEG. And if the homing endonuclease genes are neither beneficial nor detrimental to the fitness of the organism, then they will eventually spread to the entire population even if they start at a very low frequency. HEGs exist naturally in fungi, plants, bacteria and bacteriophages, but not insects. A major challenge for Burt and his colleagues is to design an HEG that will function in an animal, a goal shared by biomedical scientists looking for a new tool for gene therapy.
All these types of selfish DNA involve genes on chromosomes in the nuclei of each cell in a genetically altered pest. An alternative approach uses a selfish genetic element carried in the cytoplasm of the cell. The tool that allows this unorthodox inheritance is a kind of intracellular bacteria called Wolbachia, which is passed through the female line only (similar to mitochondria) and can manipulate the reproductive success of its insect hosts. One type of Wolbachia causes cytoplasmic incompatibility, in which the progeny of Wolbachia-infected males and uninfected females are nonviable. However, infected males breed normally with infected females. This situation provides a reproductive advantage to infected females, which can mate successfully with either infected or uninfected males. And because infected females pass on the Wolbachia infection to male and female offspring (regardless of the infection status of their mate), the frequency of Wolbachia in the population increases over time.
A group led by Stephen L. Dobson at the University of Kentucky transferred this type of Wolbachia into Aedes aegypti mosquitoes and found that the parasite spread from 20 percent to 100 percent of a laboratory population within eight generations. A strain of Wolbachia carrying an anti-pathogen gene is tantalizing to consider. But as with the other approaches, several challenges must be met before this system could be deployed. Most fundamentally, molecular biologists need to learn how to genetically manipulate Wolbachia, a process that is more difficult because the bacteria live within the cells of another organism.
These strategies are designed to spread an anti-parasite gene that would interrupt disease transmission but leave the mosquito population otherwise intact. Although entomologists view this approach as the best, most efficient means of genetic control, the earliest implementation is at least 10 years away. An alternative strategy is to use existing genetic tools to temporarily eradicate local mosquito populations. The U.S. Department of Agriculture had a similar objective in 1958 when it began eradicating the wound-infesting screwworm fly in Florida by releasing millions of radiation-sterilized adult males. The project achieved ratios of up to 100:1 sterile to normal males, so most native females mated with irradiated males to produce embryos that quickly died. Unlike the tactics above, the sterile males never passed any genes into the natural population, so factories had to produce more males for irradiation in each generation. Although it was labor-intensive, the strategy worked: Over the past 40 years, entomologists have pushed the flies to extinction from the United States to the Panama Canal.
One of the problems with sterilization is that radiation often weakens the flies to the point that they are not very competent mating partners. Furthermore, the strategy is typically only practical with pest species that allow entomologists to separate easily males from females.
Molecular geneticists have overcome these obstacles by engineering an insect that lived and mated normally, but produced progeny that could only survive on a special laboratory diet that contained the antibiotic tetracycline. The investigators use the tetracycline not to help the insect fend off bacteria, but to control the on/off switch on a lethal transgene carried by the insect.
In 1998, Walter J. Gehring and colleagues at the Universität Basel in Switzerland created the first Drosophila strains whose progeny survived only if their mothers were fed on a diet containing tetracycline. In 2005, a research team at the University of Oxford led by Luke S. Alphey reported on the first success in applying the so-called Tet-Off technique to a pest species, the Mediterranean fruit fly. If entomologists could rear such a transgenic pest strain in factories on a tetracycline-laced diet, they could release the mutants to mate with native pests and produce young that would not live without the drug. This strategy, in which both the males and females die when deprived of tetracycline, has the potential to be much more efficient than the old irradiation method.
A twist on this offspring-killing strategy was introduced in 2000 in a pair of papers from two independent laboratories, one headed by Alphey and the other by Maxwell J. Scott at Massey University in New Zealand. Both teams developed transgenic Drosophila in which only the female was dependent on tetracycline—a feature that would allow scientists to rear high numbers in a factory and then remove tetracycline from the diet in the last generation, leaving only adult males ready for release. A further advantage to this approach is that all female offspring of these males would die in the field, but the male offspring would survive and transmit the female-killing genes to some of their offspring, which would repeat the pattern.
Paul Schliekelman, a former graduate student in our laboratory who is now at the University of Georgia, developed models to examine how specific strains of mosquitoes with female killing genes are likely to affect the native populations. His work showed that a strain containing multiple copies could be 10 to 100 times more efficient at reducing the native population than a strain that killed males and females. As with the other genetic control methods discussed here, minimizing the impact of the inserted constructs on mosquito fitness is critical to success. When there are no fitness costs, more insertions result in higher efficiency because more progeny will carry at least one female-killing gene. However, as the fitness cost increases, the optimal number of insertions goes down. One nonintuitive prediction from modeling fitness costs was that the first transgenic strains released should have few copies of the engineered construct, but strains with more constructs should be released as the population declines. A Gates-funded research team led by Anthony A. James at the University of California, Irvine, is examining the possibility of using the female-killing approach for control of Aedes aegypti, the mosquito vector of dengue. But James and other investigators realize that in addition to solving the challenging technical problems involved in developing these and other types of engineered mosquitoes, they must address a host of social and ethical concerns about release of such transgenic organisms.
When we mention genetically engineered mosquitoes in conversations with our friends and colleagues, a sizeable fraction of them seem to cringe. Just the idea of such an insect scares them. Given the public discomfort with genetically engineered crops (which cannot perpetuate themselves), we expect to meet significant anxiety when people realize that our goal is to build a mosquito that outcompetes the natives.

Photograph at left courtesy of the World Health Organization. Photograph at right by Reuters/Corbis.
The Pew Charitable Fund and other groups have begun to examine the social, ecological and public-health issues that would accompany the release of an engineered mosquito. Scientists and health agencies need to educate the public about the true biological properties of genetically modified organisms. Research and regulation in this area will need to be wide open to public scrutiny. The prospective release of these mosquitoes in developing countries presents unique challenges as well. We hope that people will be more likely to accept this type of genetic engineering because of the public benefit and the fact that nonprofit groups are in charge, but to earn this endorsement, scientists need to talk about the real risk involved. The nature of that risk depends in part on the type of genetic-drive system and the disease target. For example, an engineered transposon would be unlikely to stop at national borders, so a release in one country would eventually spill into bordering countries and beyond. Alternatively, if the anti-pathogen gene were driven by an underdominance construct, then the spread would probably be much more local. (Transgenic mosquitoes are unlikely to reach high enough numbers in new areas to replace the local strain.)
Too much success could also cause trouble in the future. For example, if all the mosquitoes in a certain region were dengue-free, then a growing fraction of the population would never have been exposed to the virus. If dengue then evolved so that it could hitch a ride even on a transgene-carrying mosquito, then the human population could be vulnerable to an epidemic.
Fortunately, the increase in pesticide resistance (which would have a similar effect) has not caused such rebound epidemics, but epidemiologists do not have enough information to dismiss the possibility of future breakouts. The release of engineered mosquitoes would have to include careful monitoring and contingency plans—insecticides, different transgenic strains or vaccines (if available)—for dealing with the risk of newly evolved strains of malaria or dengue. Geneticists are already looking for ways to engineer mosquitoes that have multiple means of blocking the pathogen. (Physicians prescribe a "cocktail" of drugs to combat the AIDS virus for the same reason: A virus that mutates to overcome one drug still gets knocked down by others, preventing the spread of the drug-resistance mutation.) Given the evolutionary plasticity of microbes there is no room for complacency.
The uncertainty, effort and expense have led some scientists on the front lines in the fight against mosquito-borne diseases to oppose this line of high-tech research. Current disease-control programs are severely underfunded, and they worry that the excitement over genetic engineering will pull more money away from proven technologies such as bed nets and pesticides. This critique is valid, and funding agencies need to preserve a balanced approach. High-tech projects should not grow at the expense of these other initiatives.
You can hear both optimism and frustration at meetings where entomologists get together to talk over genetic control strategies. Scientists in this field have made great progress in the past 10 years, but major technical and social hurdles remain. In the end there will be poetic justice if biologists are able to use selfish DNA to serve the altruistic goal of improving world health.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.