First Person: Herman O. Sintim
By Fenella Saunders
Herman O. Sintim, an organic chemist at Purdue University and a Sigma Xi Distinguished Lecturer, discusses novel ways to target bacteria that cause illnesses.
Herman O. Sintim, an organic chemist at Purdue University and a Sigma Xi Distinguished Lecturer, discusses novel ways to target bacteria that cause illnesses.

DOI: 10.1511/2016.123.327
Antibiotic resistance is on the rise, and we are potentially facing a time when standard antibiotics simply won’t work anymore. Herman O. Sintim, an organic chemist at Purdue University and a Sigma Xi Distinguished Lecturer, is developing novel ways to target bacteria that cause illnesses. His approach is to prevent them from producing the toxins that lead to illness, rather than killing them. That kind of intervention avoids placing selection pressure on the bacteria, and so should reduce the chances that the bacteria will develop resistance to the intervention. Sintim discussed his research with managing editor Fenella Saunders. (A video of the full interview is available here .)

Why is antibiotic resistance becoming more widespread?
Resistance is said to occur when the dose of the drug that is used doesn’t work anymore. There are several reasons why those doses don’t work. Some could be that a type of bacteria has developed molecular mechanisms that actually make the drug ineffective. It turns out that they also have the ability to pass on the know-how to make these drugs ineffective to other bacteria. That makes it quite problematic that it only takes one bacterium to acquire resistance for that bacterium to pass that knowledge to other bacteria for them also to become resistant.
Why is the increase in bacterial resistance a serious issue?
This problem is like a time bomb, and if we don’t tackle it, it’s actually going to explode in our faces. Currently, only a small percentage of clinical cases are due to resistant bacteria, but there have been some projections that if newer drugs are not developed against these resistant bacteria that already exist, by the year 2050, death from antimicrobial-resistant bacteria could actually even surpass death from cancer. This is a big problem.
Why is resistance on the rise?
The misuse of antibiotics has certainly contributed to the resistance phenomena. A lot of antibiotics are used in farming. That leads to some resistance, because whenever any pressure is put on bacteria, the bacteria will come up with resistance. Having said that, resistance is actually an ancient mechanism. A whale researcher found that some bacteria that were present in the northern Arctic actually had vancomycin-resistant genes in them. Some of these resistant genes came about not necessarily because of the clinical use of antibiotics, but because bacteria use these molecules in fighting amongst themselves. Some are ancient mechanisms, but we have certainly helped in propagating these resistant genes.
What are common bacterial mechanisms of resistance to drugs?
An analogy is someone coming into your home who is unwelcome. The easiest way to get rid of that person is to grab that person and then throw them out of the door. Bacteria use that tactic in the sense that they have what are called efflux proteins that are membrane-bound. When the drug gets into the bacteria cell, these proteins are very promiscuous. They bind to drugs and pump them out of the cell. Other bacteria have come up with an enzyme that degrades the molecule that is supposed to work on those bacteria targets.
What alternate methods are you developing for targeting bacteria?
I am encouraged by the fact that there are more than 200 different bacteria species that live in our gut. The majority of them are actually beneficial to us. It turns out that we have learned to live with bacteria and bacteria have learned to live among one another, because different cell types communicate among themselves. The new paradigm is to come up with a strategy that would stop bacteria from producing the toxins—the virulence factors that actually make us ill—without killing bacteria. The idea is that if such pressure is not put on bacteria, then there will be no evolutionary pressure for the bacteria to develop resistance.
A few decades ago, a very interesting discovery was made that bacteria actually communicate with one another using small molecules, which are responsible for the expression of so many toxic genes. Immediately it becomes clear that if one can find a means to stop bacteria from communicating with small molecules, then the expression of these toxic genes will at least be reduced. This communication between cells is what has been termed quorum sensing.
The hope is that some of these small molecules could be used as next-generation antibiotics. These molecules are not necessarily going to kill bacteria, but they’re actually changing the lifestyle of bacteria when we change their mode of whether they actually produce these toxins.
Do approaches differ when bacteria team up to form biofilms?
Bacteria can exist as free-floating organisms. That is called a planktonic state. They can also adhere to surfaces and form a community of bacteria. That is the biofilm state. It has been estimated that about 80 percent of bacteria form biofilms. In this enclosed environment, communication among them is actually enhanced. One should actually look at a biofilm almost like a tissue: The bacteria come together and function together, just like you would get with cells in a tissue. And biofilms form almost anywhere, not just in medical settings. Every time that any solid object is put in water, bacteria will coat the surface of that object. Most of the time bacteria actually live in a biofilm state.
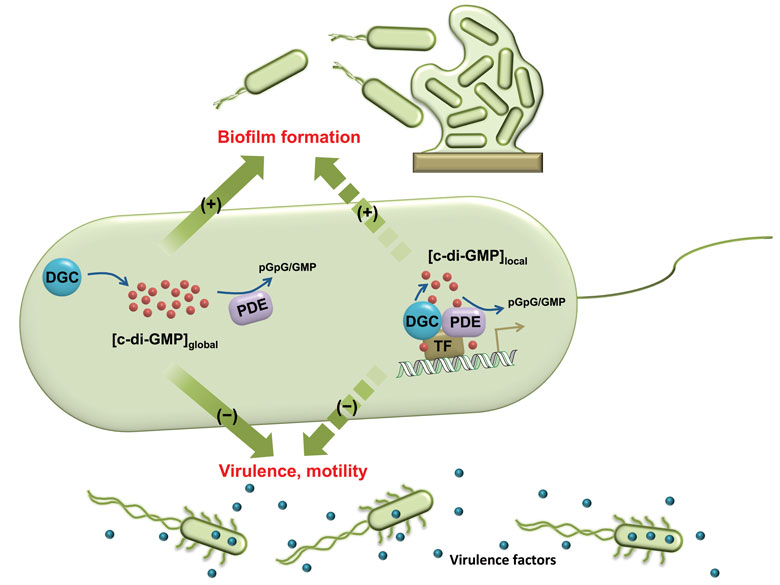
I like to refer to the biofilm state as a fortified castle where bacteria get in there and protect the cells that are embedded in the biofilm with several matrices, such as proteins, polysaccharides, and DNA. The effect of this is that when bacteria get into the biofilm state, they are between 1,000 to 10,000 times more resistant to antibiotics. Maybe if we can also get them out of the biofilm state, then we would be able to make them more susceptible to the current antibiotics that we have.
It’s interesting that the first observation of the bacteria was actually biofilm bacteria, but scientists spent decades developing drugs against free-floating bacteria. There is no drug currently being used in a clinic that actually tackles the biofilm state of bacteria.
Can other methods help with biofilms?
In collaboration with electrical engineer Reza Ghodssi at the University of Maryland, we have been interested in using electrical fields, which have been noted to dislodge bacteria. The exact molecular details of how this happens are still a hotly debated subject. But we have shown that when combining this bioelectricty effect with our signaling inhibitors, these two strategies synergize each other. The ultimate goal is to combine these in instances in which a patient has an implantable device, because biofilm formation on implantable devices is a huge problem in clinical settings.
What are the signaling pathways that you target?
One is called the autoinducer-2 pathway, which has been dubbed the so-called universal signaling pathway. We have spent the last decade actually using organic chemistry to make synthetic analogs of these signaling molecules. We have shown that some of our synthetic analogs can compete with the natural autoinducer in binding to their receptors. We have also shown that some of our synthetic analogs can have effects that are opposite to what a natural autoinducer does. We have shown that when we make synthetic analogs that are not that different from the natural autoinducer-2 molecule, we actually make molecules that dissolve the biofilms of Escherichia coli .
The power of organic chemistry is that it allows us to make libraries of molecules, and once we screen for their properties, we are able to identify ones that have the desirable properties that we want. If the signaling pathway has been elucidated and if the proteins that are involved in the pathways have been well characterized by structural biologists, then we can use computational docking methods to identify molecules that would be able to bind to these and inhibit them.
When their structures have not been solved, we look at the natural signaling molecule and make the assumption that if we make analogs that look like the natural molecule, some part of the analog would also bind. Then we use traditional chemistry to make different variants of it. I would say that is almost like shooting in the dark. We can literally make thousands of molecules that are different. That allows us to explore what we call chemical space. We make so many different variants that— because they are so different—when we then test them, we do find that some of them actually do cause antagonism.
After we made synthetic analogs, we use them to solve the crystal structure of our analogs bound to the autoinducer, and use that information to design the next generation in a rational manner. It’s almost like an iterative process. We use random methods to get our first leads, then use that for structure, and do rational designs for the next generations that are more potent.
What are the drawbacks to working with signaling molecules that are not species specific?
If there is a signaling molecule that is not species specific, that antagonist is also going to affect the beneficial bacteria in our guts. The beneficial bacteria also do very important things for us. How do you just stop the bad ones and leave everyone else? It is not very clear whether we will be able to.
The issue is confounded by the fact that in nature you don’t fight, say, E. coli just on its own. Typically bacteria are associated with other bacteria. Even if you find the means to stop one species from producing biofilms, other bacteria with which they are forming co-cultures might just come for the ride. And some illnesses also arise from the bad bacteria making the good ones become bad actors. They influence one another. That all contributes to the whole disease state. It is really a big challenge.
How do you test the signaling molecules that you develop?
Bacteria communication leads to biofilm formation, which is very easy to observe. One can stain a biofilm with dyes and use all kinds of microscopy to image it. When we make a molecule that inhibits these communications, these molecules are also going to inhibit the biofilm formation. We first grow bacteria on these microfluidic devices without our molecules and observe how the biofilms form. We can then do another experiment , in which we now add our molecules and then also observe how the biofilms form, and compare the two. If the molecules are working, we are able to conclude that our molecules are inhibiting the biofilms via the communication pathway because we have done enough experiments to show that the molecules also inhibit communication.
Another experiment that we do uses engineered bacteria that only respond to signaling molecules. Signaling molecules affect transcription factors and repressors. For example, we have a green fluorescent protein that is only made in the presence of an activator transcription factor. If that transcription factor is being controlled by a signaling molecule, then we can now add our molecules and see how that green fluorescent protein is made. That would be an indirect way of inferring that our molecule is actually interfering with the signaling.
Could bacteria also eventually develop resistance to antibiotics that use such signaling molecules?
The argument has always been that since these signaling communication networks do not affect bacterial viability, then resistance to them should be lower. If bacteria develops resistance, they’re actually put at a disadvantage, because they get out of sync with the group. But people have shown that there are mutant bacteria that are social cheaters:They themselves are resistant to the signaling molecules but they coexist with bacteria that do respond, so they can benefit from that system. It would be foolhardy to actually assume that the quorum sensing inhibition approach will not lead to resistance. What we are hoping is that it will decrease the propensity for resistance.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.