Evolution on a Frozen Continent
By David Lambert, Craig Millar, Siva Swaminathan, Carlo Baroni
Ancient-DNA studies of Adélie penguins combined with a detailed picture of a remarkable continent's geological past provide a window on evolution
Ancient-DNA studies of Adélie penguins combined with a detailed picture of a remarkable continent's geological past provide a window on evolution

DOI: 10.1511/2010.86.386
A fundamental idea in evolutionary biology is that animals are adapted to the environments in which they live, yet environments are unstable, and as environments change, animal populations respond to that change. From parasites in the gut of vertebrates, to marine animals that live in the deepest tropical oceans, to terrestrial animals that live below ground, species manage to persist and eke out a living.

Photograph courtesy of John Macdonald.
No environment is more harsh than Antarctica. During winter the continent and the seas surrounding it are dark 24 hours a day, and air temperatures are typically lower than –40 degrees Celsius. During that time Adélie penguins (Pygoscelis adeliae) live on ice floes off the coast of this huge and dramatic continent. When summer arrives, there are 24 hours of daylight and life becomes easier, but mean temperatures can still be –20 degrees Celsius. Penguins then come ashore on the continent to construct nests, mate, raise their chicks and finally return to the sea. The appropriateness of Adélie penguins to their conditions of life is obvious. For example, their hydrodynamic body shape is very similar to that of seals and is well suited to the marine environment in which both animals live. Physiological and metabolic adaptations enable them to withstand the very cold temperatures of their terrestrial and aquatic environments.
However, we know that when environments change—for example, when temperatures increase or decrease—many animal species, rather than adapt to their new conditions, simply move to stay within their preferred temperature limits. When times were colder than they are now, such as in the cold phases of the Pleistocene period (2.5 million to 11,700 years ago), it seems that the Antarctic might not have been a good place for an Adélie penguin to live. Nearly all of Antarctica would have been covered in snow and ice and because Adélies breed only in ice-free areas, the usable breeding grounds for the species would have been very restricted. There is no evidence that Adélie penguins moved in response to this altered environment and went to warmer, ice-free areas to breed, as we might expect.
In a world increasingly concerned with global climate change and particularly the likelihood of rising average temperatures, spare a thought for Adélie penguins. Only the emperor penguin and the Adélie nest solely on the Antarctic continent or islands close to it, and the Adélie is the one penguin that breeds only on ice-free areas of the continent. (The emperor breeds on the ice itself.) Other species, such as the closely related chinstrap and gentoo, breed mainly on sub-Antarctic islands. If global temperatures increase, Adélie penguins will not be able to simply migrate to a colder part of the continent—they will have nowhere to go because they already live in the coldest place on Earth. This makes the Adélie penguin an ideal species for studying adaptive evolution in the context of global climate change.
In Antarctica, the Pleistocene epoch was distinguished by the repeated expansion and collapse of huge marine-based ice sheets (thick floating platforms of ice that form where glaciers project into the sea), as well as by fluctuations in the volume of ice on the Antarctic landmass. These changes would have certainly caused large-scale disruptions to animal habitats and populations. Adélie penguins generally breed in large colonies on ice-free areas close to the sea. For example, Ross Island, situated at the edge of the Ross Ice Shelf, is presently home to several hundred thousand breeding pairs of birds. In Antarctica, even small fluctuations in the volume of ice can have a significant impact on the number and extent of ice-free areas. At the Last Glacial Maximum (LGM, about 25,000–18,000 years ago), the entire Ross Sea coastline was uninhabitable to Adélie penguins because of the extent of the Ross Ice Shelf; Ross Island itself was almost 900 kilometers inland from the edge of the ice and the open sea. Such glacial events undoubtedly influenced penguin abundance, distribution and genetic diversity.
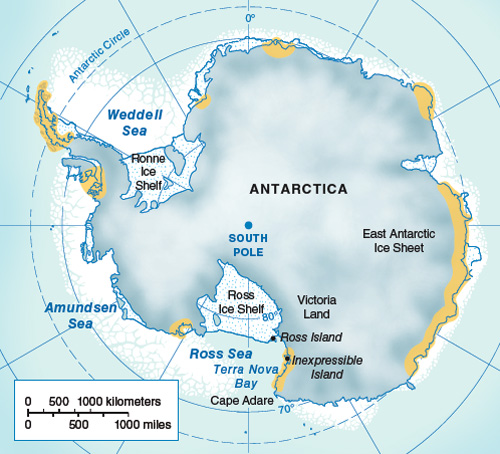
Illustration by Barbara Aulicino.
As suggested by the observations of Robert F. Scott at the beginning of the last century, Antarctica during the LGM was covered by an expanded ice sheet advancing onto the continental shelf, and coastal areas were buried by hundred of meters of ice. In the Ross Embayment, the coastline became ice-free only about 8,000 years ago, after the retreat of the LGM ice sheets and of their fringing ice shelves. Moraines are deposits of soil and rock debris left behind by retreating glaciers; their locations in ice-free areas document fluctuations in the extent of glaciers. On the low, rocky coastlines of the Ross Sea, raised beaches and marine terraces of the Holocene epoch (reaching back from the present to about 11,000 years ago) resulted from sea-level changes caused by the large amounts of water released by retreating ice sheets, together with isostatic rebound, the rise of land masses previously depressed by the huge weight of the ice during the LGM of the Antarctic coastal belt.
Adélie penguins are the dominant terrestrial species in Antarctica. They have been described by ecologist David G. Ainley as a bellwether of climate change, in part because they are adapted to a narrow habitat optimum between too much sea ice and not enough. Certainly Adélies have lived through many dramatic changes in temperature. Over the past two million years there has been a series of ice ages in Antarctica and elsewhere. Evidence of these events, directly preserved in the East Antarctic Ice Sheet and described in our work and that of others, indicates that ice ages occurred regularly every 41,000 years in the early Pleistocene and about every 100,000 years since 430,000 years ago. During the last glacial-interglacial transition (circa 18,000–12,000 years ago), climate change in the Antarctic was the most extreme on Earth, with the average temperature rising by about 13 degrees Celsius, as documented by ice cores. Since that period Adélie penguins have been numerous in Antarctica, both in terms of total biomass and of the numbers of breeding individuals. David Ainley estimates that the current population of Adélie penguins is about 5 million individuals.
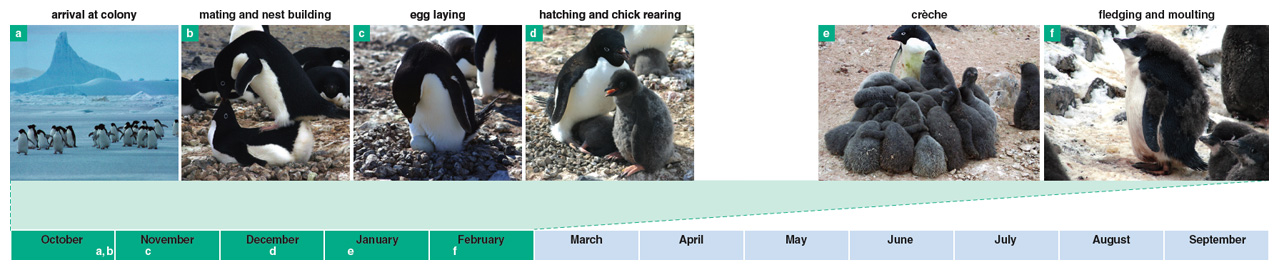
Figure 3. The breeding season for Adélie penguins on Ross Island begins in late October, the start of the Antarctic spring. During the other seasons of the year they likely travel as far as a few hundred kilometers north of the Antarctic continental shores before returning to their breeding grounds. Colonies range in size from a few dozen pairs to over 200,000. Female Adélies lay two eggs, and then both parents share the chores of incubating the eggs and foraging for food.
Adélies breed in colonies at ice-free sites around the coast of Antarctica and on some islands off the Antarctic coastline. On the continent these colonies have a patchy distribution, presumably in part at least because of the criteria the birds use to identify breeding sites. Adélie penguins construct their nests from pebbles of a very specific size range. In areas where meltwater may wash over the breeding grounds, the nesting stones act to keep the developing eggs and chicks above the surface where they can remain dry. Ainley has identified common features of Adélie breeding colonies, including close proximity to pack ice during the winter and early spring, and accessibility to the sea by walking during summer. In 1915 the British explorer George Levick noted the apparent importance of locations in which high winds were common, suggesting that the winds swept the ground and pebbles of snow. Adélie penguin colonies vary hugely in size, from less than 100 individuals to the largest colony in Antarctica at Cape Adare. This colony lies at the mouth of the Ross Sea and comprises about 220,000 breeding pairs (as reported in a personal communication from Phil Lyver, zoologist with New Zealand’s Landcare Research organization). Currently there are 24 known breeding colonies along the coast of the Ross Sea and a large number of abandoned rookeries between Terra Nova Bay and Ross Island.
Adélie penguins begin a regular annual cycle of breeding during the Antarctic spring, with males typically arriving at Ross Island colony sites in the last week of October and early November, on average four days earlier than females. Adélie penguins are generally monogamous. Males typically begin breeding at 5 to 7 years, females at 4 to 6 years. In mid November, females usually lay two eggs and then leave to feed at sea for 8 to 14 days. During that period, males incubate the eggs. The adults then change places and the nonincubating bird returns to the sea to feed.
High breeding-site fidelity is characteristic of the species. According to data collected by Ainley, 96 percent of breeding birds have been recorded nesting at their natal colony and 77 percent bred within 100 meters of their natal site. However, it is clear that environmental conditions can dramatically interfere with this generally high level of return. For example, the ability of adults to feed at sea and provide sustenance for their growing chicks can be disrupted by massive icebergs, which if grounded near breeding colonies may greatly increase the distance adults must travel to find open sea.
As chicks get older they are recognised by their parents, and after about three weeks they begin to leave the nest and mix with other chicks in the colony. Chicks become independent of the nest at this crèche stage. The larger size of chicks at this time requires both parents to forage at sea. On returning, parents are able to recognise their chicks among the large number of juveniles in the crèche by their calls. Chicks eventually fledge in early February.

Photograph courtesy of Carlos Olavarria.
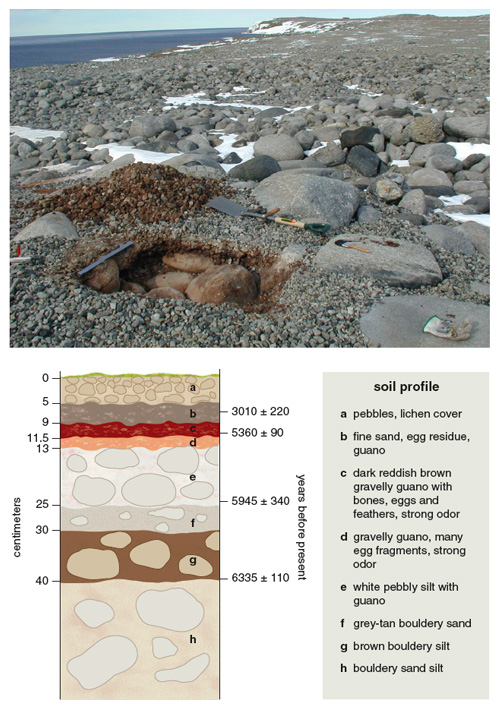
During the breeding season, colonies are grim places in which predation of eggs and chicks occurs at high levels. Adélie penguin remains lie on the ground for seasons and are well preserved by the cold, dry Antarctic climate until they are eventually buried by the remains of new nests and other deposits. Penguin guano seeps through the permeable, pebbly nests and accumulates at their bases. Each successive occupation of a colony creates a layer made from nest pebbles, penguin remains and guano to form ornithogenic soils. The extent and thickness of these organic layers is a function of the size, age and persistence of the colony—the older the colony, the thicker the accumulation of pebbles and guano. Hence, ornithogenic soils contain a well-developed stratigraphic record of Adélie penguin colonies: guano, bones, eggshell fragments and dietary remains (fish otoliths, bones and teeth, and squid beaks).
Members of our research team regularly excavate ancient colonies to recover these precious remains for radiocarbon dating and DNA analysis. Hundreds of such radiocarbon-dated penguin remains collected from ornithogenic soils document the past availability of ice-free coastal sites suitable for nesting and breeding. Along the Victoria Land coast, two recent sets of radiocarbon dates indicate a Late Pleistocene occupation at about 45,000 to 25,000 years ago and a later Holocene recolonization of these coastal areas. Furthermore, dates from ornithogenic soils, together with other datable organic materials, provide data for reconstructing the retreat of glaciers in coastal areas after the LGM and the subsequent emersion of coastline starting about 8,000 years ago during the Holocene period.
Photograph courtesy of Carlo Baroni. Illustration by Barbara Aulicino.
Ornithogenic soils in the vicinity of currently occupied colonies suggest that the penguin population expanded and contracted repeatedly during the Holocene. Abandoned nests are easily identifiable as accumulations of well-sorted pebbles. After the abandonment of these breeding sites, scouring winds leave a concentration of pebbles at the surface that protects the guano layers below from erosion.
Abandoned penguin nesting sites in areas where Adélies do not currently nest have been recognized as relict colonies and are common landscape features along the Antarctic coasts. The accurate geomorphologic survey of ice-free areas led to the discovery of tens of relict colonies. Applying the usual techniques of archaeological research, such as precise stratigraphic excavation of penguin settlements, we have been able to identify phases of occupation and abandonment of the relict colonies, with the phases separated by mineral layers such as aeolian deposits, sand and gravel of colluvial or periglacial origin, and so on. From these data we are able to reconstruct the history of penguin populations.
In the Ross Sea area, penguin re-colonization is intimately associated with elephant seal (Mirounga leonina) presence and distribution related to warmer-than-present conditions in the area. After the deglaciation and until about 4,000 years ago, the two species coexisted along the Victoria Land coast, indicating that there was less sea ice than at present, a state preferred by elephant seals, but still sufficient regional pack ice for Adélie penguins. The period between 4,500 and 2,500 years ago registered the ‘‘penguin optimum,’’ a period of particularly favorable environmental conditions for penguins (perhaps because an increase in pack ice resulted in more favorable foraging ecology). During this period there was a more extensive settlement of these coastal areas by penguins. Now there are fewer penguin colonies, a reduction associated with a decline in the populations of elephant seals. The sudden decrease of penguin colonies and populations occurred approximately 2,500 years ago.
Between 2,300 and 1,100 years ago, the establishment and persistence of sub-Antarctic climatic and environmental conditions inhibited the establishment of Adélie penguin colonies, as documented by the contemporary spreading of elephant seals. This period represents the greatest sea-ice decline (and probably the warmest ocean and air temperatures) in the Ross Sea in the last 8,000 years. In the last 1,000 years, penguins populations expanded again and elephant seals abandoned the area.
This detailed picture of the glacial history of Antarctica and changes in the number and distribution of Adélie penguins provides the necessary backdrop for our investigation of evolution itself. In principle, by comparing DNA sequences from the same genomic regions in individuals of one species that lived at different times, it should be possible to determine what evolutionary changes have occurred over time and then to measure the rate at which the changes in DNA occurred. To put the importance of the rate measurement in context, we might say that measuring the speed of evolution is as important to biologists as measuring the speed of light was for physicists. Knowing how fast DNA changes over time has relevance for many disciplines from forensics and evolutionary biology to taxonomy. It enables biologists to calibrate the timing of important evolutionary events in the history of life and more generally to provide a ”clock” for evolution itself. So, for example, if one compares DNA from modern (living) individuals and those that lived 1,000 years ago, 2,000 years ago and so on, the changes over these time periods can reveal the speed—and potentially even something about the nature—of evolution itself. But to do this analysis, we need serially preserved samples of precisely known age from which ancient DNA can be recovered. Samples from living animals of the same species—from time zero if you like—are also necessary. The woolly mammoth and Neanderthals are not suitable subjects for such evolutionary studies because no modern populations of these species exist. And of course ancient DNA is not available for all species. DNA is best preserved in low temperatures and dry conditions. Species that live in equatorial regions and those that inhabit wet environments are unlikely to be good targets for such work. Therefore it is not surprising that much ancient-DNA research has focused on animal species inhabiting the polar and temperate regions. These studies have dramatically improved our estimates of rates of molecular change, our understanding of the biology of ancient populations and our estimation of the amount of genetic variation that has been lost or gained in these species over time.
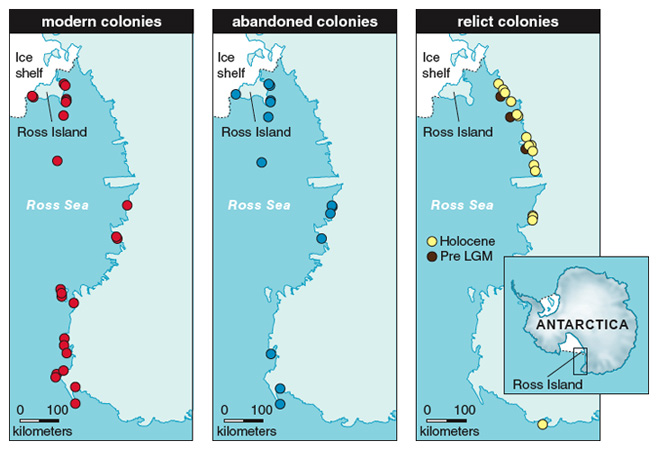
Illustration by Vivian Ward/School of Biological Sciences, University of Auckland.
Evolution consists of changes in the genome, some in DNA regions under selective control, others in neutral sequences. Many evolutionary studies concentrate on neutral sequences because those under selective control are responsive to episodic environmental changes and are unlikely to change at a constant rate.
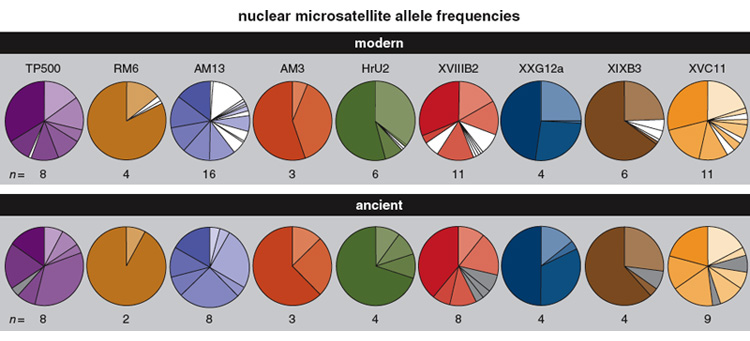
Evolution occurs in the mitochondrial genome as well as the nuclear genome. Mitochondrial DNA has been a prime target for ancient DNA studies because there are hundreds to thousands of copies of mitochondrial DNA present in each cell, versus one copy of the nuclear genome; because the rates of mitochondrial DNA change are typically high; and because ancient mitochondrial DNA sequences can be up to 300 base pairs long, whereas nuclear sequences are virtually never longer than 150 base pairs. Nevertheless, owing to the extraordinary quality of ancient Adélie penguin DNA preserved in the cold dry storage of the Antarctic, we were encouraged to attempt to reconstruct a sample of nuclear gene loci using subfossil remains of breeding birds from Inexpressible Island in the Ross Sea. Using nine nuclear microsatellite DNA loci (microsatellites are sequences of a few repeating base pairs of DNA), we genotyped an ancient population of Adélie penguins from a single stratigraphic layer using two radiocarbon-dated bones aged 6,082 ± 55 years and 6,092 ± 60 years. Subfossil bones from this population were excavated using a stratigraphic method that allowed the identification of individuals even within the same layer. After comparing the allele frequencies in the ancient population with those recorded from the modern population at the same site in Antarctica, we reported significant changes in the frequencies of alleles at four of the nine loci we examined, demonstrating microevolutionary change over the approximately 6,000-year period. This outcome was the first demonstration of a change in the frequency of alleles in the nuclear genome over such a geological time frame.
Because most microsatellite DNA loci are noncoding, natural selection is unlikely to have significant influence on the evolution of such genes. From the findings of a large number of excavations carried out in the Adélie penguin colony at Inexpressible Island, it appears that this population has been large over the 6,000-year period of this study. However, we cannot exclude the possibility that population bottlenecks may have occurred during this time that significantly affected gene frequency changes.
Unlike other genetic markers that typically mutate by single nucleotide changes, the predominant type of mutation for microsatellite DNA loci is changes in the lengths of their repeat tracts, which arise due to slippage during replication and to unequal exchange of genetic material during recombination. Certainly it is widely recognised that microsatellite DNA alleles tend to increase in length over time, at least when alleles are short and phylogenetically young. Consequently, we compared the length of alleles in ancient and modern populations and showed that the ancient population has significantly shorter microsatellite DNA alleles at four of the nine loci examined. Three of the remaining loci showed longer alleles in the modern population, although the differences were too small to be statistically significant. In addition, the ancient population is characterised by short private alleles (alleles that are unique to a population) whereas the modern population reveals a larger number of private alleles that are generally among the longest variants at each locus. This result suggests that we have identified the mutational processes—errors during DNA replication that result in length changes—that have contributed to the microevolutionary change we detected.
In addition to our findings about nuclear DNA, we have been able to estimate rates of molecular change by studying changes in mitochondrial DNA sequences using many serially preserved subfossil remains of Adélie penguins. The rates we derived are higher than many other previous estimates, yet they are generally robust to different methods of analysis. The rate of molecular evolution of the HVR I region, determined using DNA sequences from 162 subfossil bones of known age spanning a 37,000-year period, was 0.86 substitutions per site per million years (with a 95 percent confidence interval of 0.53 and 1.17).
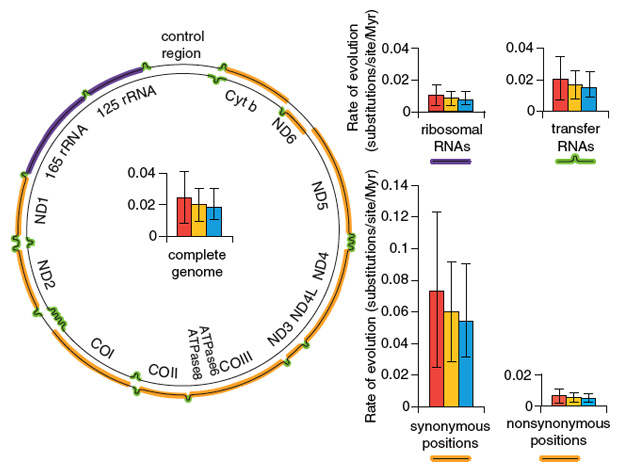
We have also recently been able to sequence complete mitochondrial genomes of Adélie penguins up to 44,000 years old. Using these data, we have been able to estimate rates of molecular change for the entire mitochondrial genome, as well as rates for components of the genome, such as sequences coding for transfer RNAs and ribosomal RNAs, as well as protein-coding regions. For the latter, we have been able to determine rates for synonymous sites (the variations found do not change the amino-acid sequences of the coded proteins) and nonsynonymous sites (the amino acid sequences coded by the alleles are different). These rates are all generally high, indicating a rapid rate of molecular evolution over geological time.
Direct observations of mutation rates (obtained by studying modern samples) and longer-term molecular rates of change (obtained by comparing modern and ancient DNA) will help us understand how fast genetic variation arises and how fastidiously it is preserved. Are the two related? Perhaps the high evolutionary rates we discovered are elevated because of high mutation rates? We have been able to study both mutational and evolutionary processes using Adélie penguins as a model organism. By taking blood samples from adult breeding birds and their chicks, we have been able to detect new mutations and to analyze whether they are germline mutations inherited across generations. Our results have been surprising. We sequenced DNA from the mitochondrial HVR I region from penguins comprising a large number of penguin families. Our sample consisted of more than 900 chicks and both parents of each chick. We recorded a total of 62 germline heteroplasmic mutations (those in which two DNA variants were recorded at the same position in the mitochondrial genome within the same individual) in these families. These variants were detected in mothers and also in their offspring, consistent with maternal inheritance. The data give an estimated mutation rate of 0.55 mutations per site per million years (95 percent confidence interval of 0.29 and 0.88) after accounting for the persistence of these heteroplasmies and the sensitivity of current detection methods. Importantly, this rate is not significantly different from rates of evolutionary change we have estimated using serially preserved subfossil remains of Adélie penguins. This study suggests that molecular rates of change—either mutational rates occurring between successive generations, or evolutionary changes over much longer periods—are essentially no different. The challenge now is to recover much longer regions of the nuclear genome and to focus on regions that may help these remarkable birds respond to the challenges they will encounter in the face of a changing climate.
Playing on the famous metaphor of ecologist George Evelyn Hutchinson, the frozen continent of Antarctica is a remarkable “ecological theater” in which Adélie penguins are central characters performing in an “evolutionary play.” As the nature of the theater changes with every ice age, or in our case with every interglacial warming period, the storyline of the play changes. But will it always do so? As the planet warms, Adélie penguins have nowhere colder to go to escape the change. They must either adapt or perish. Researchers can expect to read the script of this drama in the allelic variations of the penguins’ DNA.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.