Angiogenesis, Aging, and Alzheimer’s Disease
By Charles T. Ambrose
Can a form of treatment that promotes the growth of new capillaries, improving blood flow in the brain and elsewhere, ease old age and Alzheimer’s disease?
Can a form of treatment that promotes the growth of new capillaries, improving blood flow in the brain and elsewhere, ease old age and Alzheimer’s disease?

DOI: 10.1511/2016.119.82
During the past several decades, research in aging and dementia has been dominated by the amyloid cascade theory, which identifies the deposition of amyloid-β in the brain as a possible cause of Alzheimer’s disease. Research with this focus has not, however, produced the expected therapeutic magic bullet for Alzheimer’s disease—at least, not one that is widely accepted. Various investigators have highlighted this disappointing result and have urged new approaches to the disease.
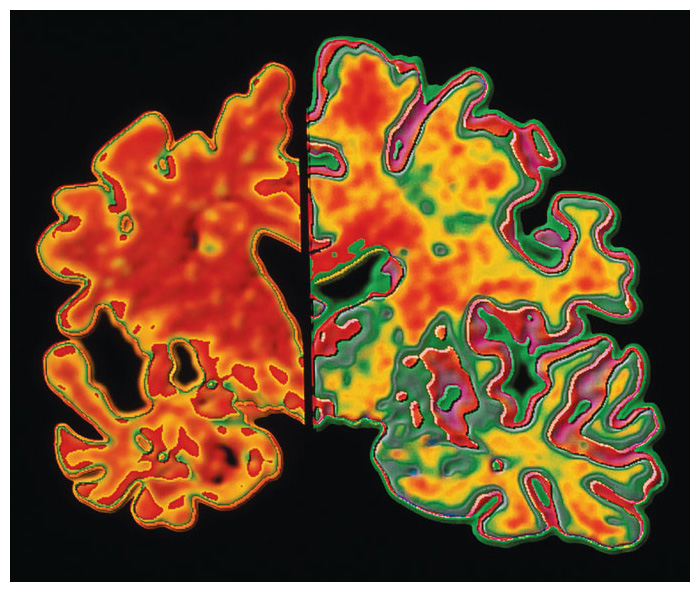
Diagnosing Alzheimer’s disease is not easy, because this neurodegenerative condition often affects a person’s behavior and mental status in subtle ways. A clinical diagnosis of Alzheimer’s disease is often made in an elderly person who shows signs of impaired cognition; however, the one biomarker that allows for a definitive diagnosis is the accumulation of amyloid plaques and neurofibrillary tangles in the brain, which can be observed only in the histological examination of a brain at autopsy. Even this sign is not absolute: In some cases, the histological changes that define Alzheimer’s disease are also found in cognitively normal persons and are absent in the brains of some persons exhibiting cognitive decline. This paradoxical finding has led some investigators to question the supposed etiology of Alzheimer’s disease. It is worth considering is whether there is some underlying, as-yet unrecognized pathology that can account for this dementia, and perhaps for others as well.
A diminished microcirculation is a common histological change in the brains of aged persons (and of old animals) as well as in their muscles and other organ systems. Microcirculation is commonly termed and measured as capillary density , a reduction of which has been reported in the brain of those with Alzheimer’s disease, most of whom, of course, are aged.
An important factor in the development of Alzheimer’s disease may be a reduced cerebral microcirculation. This idea has appeared occasionally in the literature in various forms. For example, in 1993 Jack de la Torre postulated an impaired cerebral blood flow in Alzheimer's disease patients due to “twisted, looped, and kinked” capillaries often found in their brains. It seems plausible that these distortions precede the age-linked decline in capillary density. But his idea and related ones never gained the ground swell of the amyloid-β theory. Perhaps a genetically determined, age-linked reduced capillary density in the brain may seem too simple an explanation to be true. It doesn’t have the intellectual appeal of more complicated theories.
A vast literature now exists on angiogenesis (the formation of new capillaries), and angiogenic factors (for example, vascular endothelial growth factor, VEGF) in various disease conditions, such as tissue repair, cardiac insufficiency, solid tumors, and so on. In 2011, the researchers Peter Carmeliet and Rakesh Jain speculated that “the revascularization of ischemic tissues would benefit millions, but therapeutic angiogenesis remains an unmet medical need.”
Perhaps a conceptual weakness in the study of Alzheimer’s disease has been to regard it as a condition distinct from aging in general, instead of merely one presentation of aging. This reassessment is expressed in the angiogenesis hypothesis , which proposes that old age is in part a deficiency condition due to an age-associated decline in angiogenic growth factors, which in turn leads to a reduced microcirculation throughout the body. This deficiency accounts for the early symptoms and signs of old age—the so-called lesser ailments such as diminished stamina, progressive muscle weakness, intolerance of cold, and minor memory lapses. The last may predate the development of senile dementia.
The age-linked reduction of angiogenic factors is genetically determined; in this, it is analogous to certain hormones whose blood levels also decline with age. The angiogenesis hypothesis does not exclude other influences contributing to the aging process, for indeed it's generally assumed that most persons grow old due to several causes.
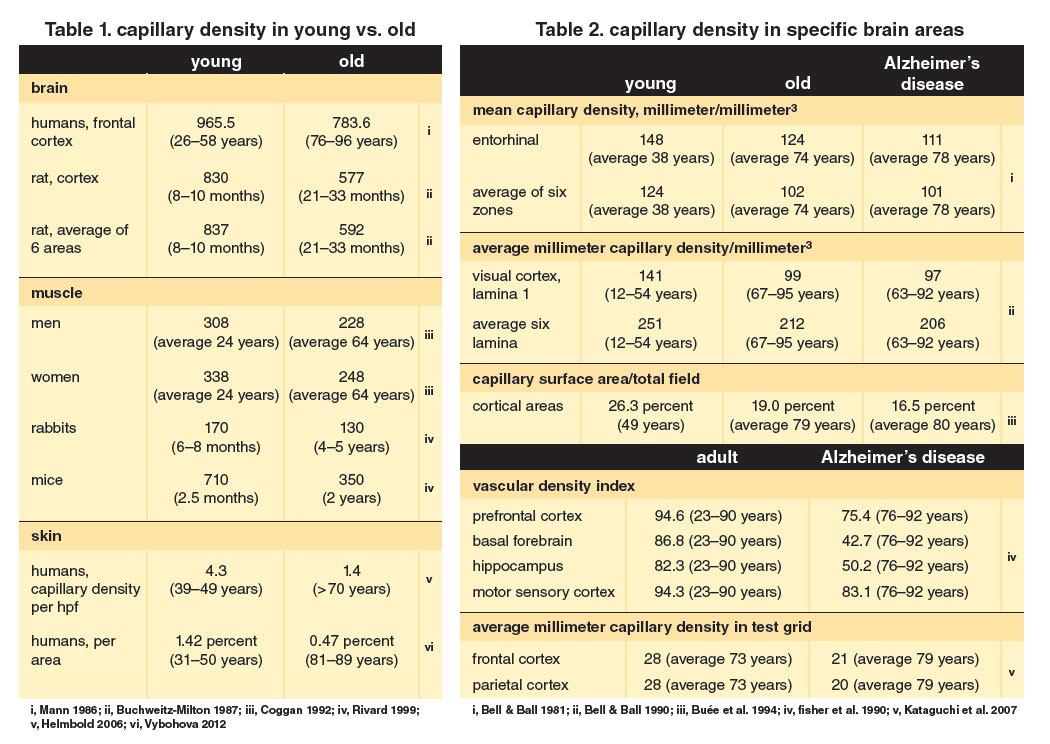
Abundant data from published articles document a reduced capillary density in the brain, muscles, and skin of aging animals and elderly persons (Table 1). Nearly a dozen reports show a reduced capillary density in Alzheimer’s disease patients like that for comparable elderly persons (Table 2). As noted before, angiogenic growth factors determine capillary density. Many papers report age-associated declining levels of angiogenic growth factors in these organ systems (Table 3). Such information from over 40 studies suggests that a reduced capillary density and declining levels of angiogenic growth factors are invariable changes in the body during aging—a linked phenomenon comparable to Walter Cannon’s concept of homeostasis throughout life. Scanning the data in these two tables and those in recent reviews may be more convincing than reading generalizations in a text about reduced capillary density and angiogenic growth factors with aging.
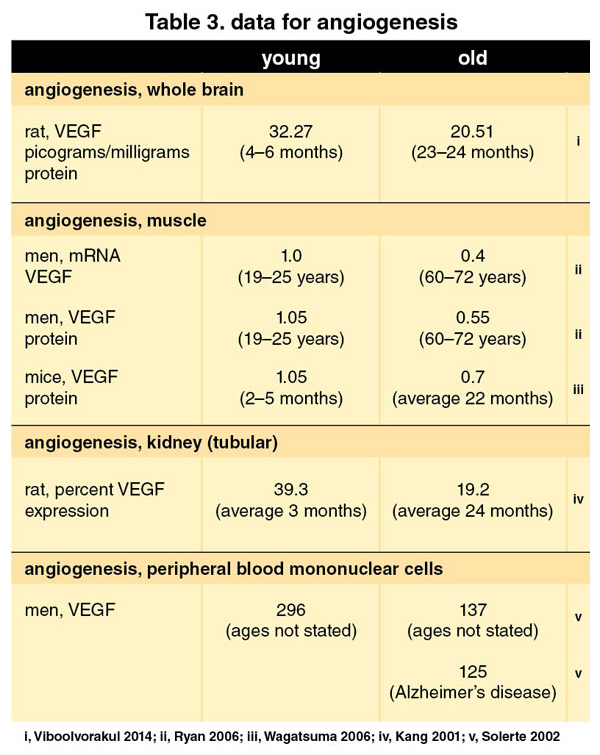
Of course, all the data in these tables can only establish a temporal association between (a) aging and (b) a reduction of capillary density; they cannot show how (a) and (b) are related, or even whether they have any bearing on each other. Convincing evidence would be provided if some specific symptom or sign of old age could be relieved or delayed by the administration of angiogenic growth factors.
Recombinant forms of VEGF and fibroblast growth factor-2 have been given in studies both to patients with cardiac ischemia (insufficient blood flow to the heart) and those with intermittent claudication (leg cramps caused by temporary blockage of blood supply during exercise). Both groups reported an initial benefit, but long-term assessments are lacking.
During old age, the regenerative capacity of tissues and organs in the body is crucial for health. Numerous studies have shown this capacity to be influenced by blood-borne factors present in young mice but absent in old ones. These investigations have logically focused on those factors that decrease with aging. Early research concerning regeneration involved parabiotic mice—that is, two animals surgically sutured together so that their circulatory systems commingle. A young mouse (2–3 months) and an old one (19–26 months) united together represent heterochronic (different-aged) parabiosis. Mice of the same age joined together form isochronic parabionts. Humoral factors flow from one into the other via the linked circulation.
In experiments by Irina Conboy and her group at Stanford University, the hind limb muscles of each surgically joined mouse were injured five weeks after establishing parabiosis. Five days after the injury, they observed robust regeneration with new myotubes in both the young-young pair and the young-old pair, but not in the old-old pair. The authors interpreted the regeneration in the old mice in heterochronic pairing as being due to “activation of resident [myoblastic] progenitor cells,” stimulated by “systemic factors” in the young blood.
Also at Stanford University, Joseph M. Castellano and his colleagues reviewed the rejuvenation effects of heterochronic parabiosis on seven specific organ systems of the older mice: brain, skeletal muscle, heart, bone, liver, pancreas, and hair follicles. They reiterated the “likelihood that growth-promoting molecules are abundant in young plasma.”
A logical extension of parabiotic studies has been experiments in which old mice receive plasma from young mice. For example, in the laboratory of Saul Villeda at the University of California in San Francisco, old mice were injected with 100 microliters of young plasma eight times in the course of three weeks; afterwards they showed improved “hippocampal-dependent learning and memory.” Extracellular electrophysiological recordings on hippocampal slices of these old mice revealed an increase in synaptic plasticity, for which the investigators credited “pro-youthful factors” in the young blood.
At Harvard University’s department of stem cell and regenerative biology, Lida Katsimpardi tested aging mice with a growth differentiation factor known to decline with age, recombinant GDF11. Daily injections of this factor increased blood vessel volume by 50 percent, from 47 to 71 cubic micrometers. The number of branches on blood vessels also increased by 21 percent, suggesting, of course, angiogenesis. The authors speculate that “increased blood flow might result in increased neural activity and function, opening new therapeutic strategies for treating age-related neurodegenerative conditions.” The precise mechanism of this increase has not been elucidated but may involve stimulation or release of endogenous angiogenic growth factors.
Skeletal muscles have also been rejuvenated in aged mice during heterochronic parabiosis, as well as in old mice receiving injections of recombinant GDF11. In these studies, however, the research group, led by Manisha Sinha at Harvard University, has focused more on restoring the “genomic integrity” of aged muscle stem cells than on investigating the underlying vascular mechanism. This work, along with other research from the Harvard Stem Cell Institute and the department of stem cell and regenerative biology, is the basis for clinical trials with GDF11, which are anticipated to begin within a few years. Already under way at Stanford University is a related clinical trial in which plasma from young persons is infused into subjects with mild-to-moderate Alzheimer’s disease.
Levels of cerebral capillary density in persons with different cognitive abilities could be determined from special brain collections, such as the one that has become known as the Nun Study.

In 1986, David Snowdon, then at the University of Minnesota, began research on aging in a cohort of over 600 nuns, who were assessed during their later years for their cognitive status. After their deaths, the brains of over 500 of these individuals were obtained for study of histological signs of Alzheimer’s disease and other neurological conditions. Evaluating the capillary density in two groups of brains—one from nuns who had remained cognitively normal to the end of their lives, and the other of nuns showing evidence of dementia—would indicate whether a correlation could be made with the donors’ cognitive states.
There are other cohorts of brains appropriate for research relating cognition and capillary density. Of course, any such collection might require permission from a medical ethics committee for use in a new histological study, but at least the more arduous tasks of soliciting subjects for autopsy, gaining their informed consent, procuring the brains at autopsy, and so on, would be past.
As discussed earlier, the parabiosis studies in mice have demonstrated that humoral factors in young blood can indeed exert reparative effects, such as new myotubules and improved learning and memory—repairs that to some extent ease and delay the aging process. We postulate, therefore, that reduced levels of angiogenic factors in older people may account for the hallmarks of aging, and that they are much like other age-associated endocrine factors.
For the angiogenesis hypothesis, the important aspect of old age is not its chronology but its signs and symptoms. Relieving the hallmarks of old age or delaying their onset by long-term treatment with recombinant angiogenic factors would constitute the ultimate affirmation of the hypothesis.
In previous publications I have discussed therapeutic angiogenesis, including the nasal route for the regular administration of recombinant angiogenic factors. Further proof of the angiogenesis hypothesis may be needed before considering clinical trials. Various research groups already have the microscopic competence to test the validity of the angiogenesis hypothesis by measuring capillary density in various tissues. As discussed earlier, an example would be a cohort of aged brains, such as that of the Nun Study, where the cognitive status of each brain donor at death is known. As assessment of capillary density in the brains of two groups—cognitively normal versus demented—could validate or refute the hypothesis and help decide whether to pursue clinical trials with angiogenic growth factors. Similarly, a comparison of capillary density in an upper thigh muscle of strong and weak older persons paired by age might provide for an informative analysis.
This invitation to test the angiogenesis hypothesis is open to interested investigators. Until its firm support is at hand, therapeutic angiogenesis remains an unapproved but tantalizingly tenable treatment for old age, including possibly for Alzheimer's disease.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.