In the Thick of It
By Fenella Saunders
Ruffling cell membranes tamp down and spread out in stickier fluids, helping cells move faster through mucus and other body environments.
Ruffling cell membranes tamp down and spread out in stickier fluids, helping cells move faster through mucus and other body environments.

Cells that are sitting in a lab are usually immersed in a fluid that’s about as thick as water, but cells in the body are hardly ever exposed to fluids that thin. “There are a lot of gooey fluids in your body,” says Yun Chen, a biomedical engineer at Johns Hopkins University. If researchers want to understand how cells really behave under body conditions, they need to make the lab conditions similar. Previously, researchers thought that cells would move or divide more slowly when their surrounding fluid is thicker. But Chen and her colleagues have found, surprisingly, that cells move faster.
The researchers knew from other studies that the surface on which cells are grown can affect how the cells express genes, but no one had yet closely investigated the effect of the fluid environment on cell behavior. Chen and her team studied different kinds of cells: fibroblasts from connective tissue, macrophages, and kidney cells, among others. Almost all cell types in the body have the ability to move under some circumstances, such as when they are fighting infection or undergoing tissue repair.
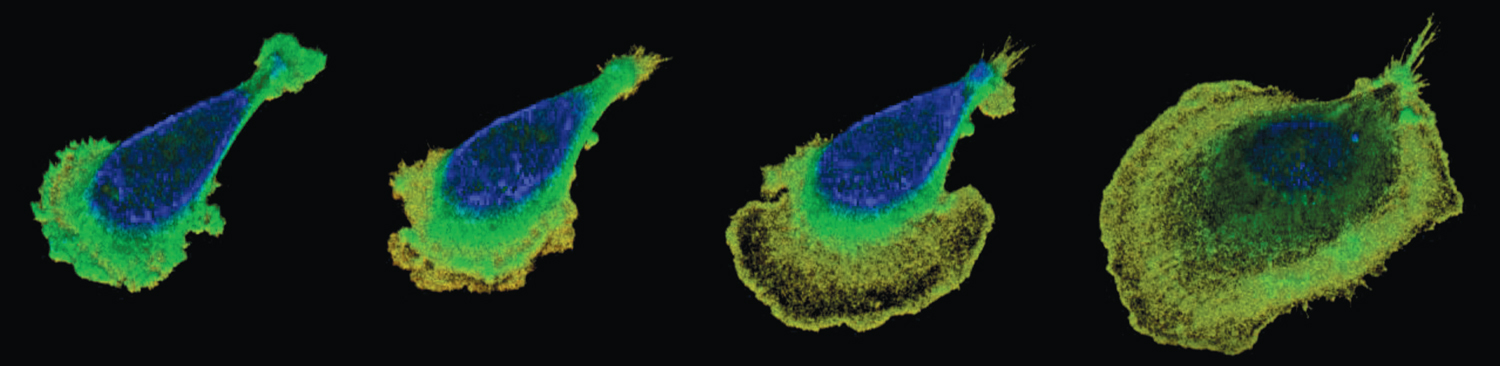
Images from Pittman, M., et al., Nature Physics 18:1112.
As Chen and her colleagues reported in the September 2022 issue of Nature Physics, the team used interference reflection microscopy to image the cells, and they developed intricate image processing and analysis software to see what happens when cells are put into a viscous medium. They found that when cells are in a fluid the viscosity of water, their membranes gently ruffle, fluttering up and down in place. But as soon as viscosity increases, the cells spread and flatten out dramatically, and their speed increases twofold (see figure above).
Chen explains that inside of cells there is a cytoskeleton, a scaffold that determines the cell’s shape. The cytoskeleton is changing dynamically, as polymer rods called actin filaments are built up and push on the cell membrane in the direction the cell is trying to move. But the membrane, Chen notes, has its own tension that resists pushing. If the membrane is taut, the actin filaments will bend, which also pushes the membrane upward, causing the ruffling movement of the membrane. “It’s like pushing a rod against a wall. In the beginning that might be okay, but if you keep ramming it, your rod will buckle,” Chen says. “Ruffles are just an interplay between the protruding forces and the membrane tension.”
From Pittman, M., et al., Nature Physics 18:1112, courtesy of Yun Chen.
But factors change when the cell is in a higher viscosity fluid. The first factor affects a protein called integrin that sticks out across the cell membrane. “Integrin is like the little hooks on Velcro,” Chen says. “It comes out of the cell and will hook the cell onto the substrate.” Where the cell is hooked is known as a focal adhesion. When the cell is being pressed down by a higher viscosity fluid, the integrin has an easier time creating adhesions on the surface. Those adhesions help to stabilize the actin filaments so they buckle less, but the adhesions also reduce the tension on the cell membrane. “The contact is taking some of the load of the tension on the cell membrane and sharing it with the substrate,” Chen explains. “The membrane is not as taut anymore, so the actin filaments can keep pushing forward, and that’s when you see the cell spread.”
The focal adhesions with the hooklike integrin proteins attach and detach, pressing on the surface and providing a way for the cells to move at a greater speed (see figure below). “You can imagine focal adhesions as the little feet of the cells. The cells put down their little feet, and then they lift the feet when they move forward,” Chen says. “If you have a lot of focal adhesions, you can imagine it as if you have a lot of feet moving in the same direction. That will help the cells move faster.”
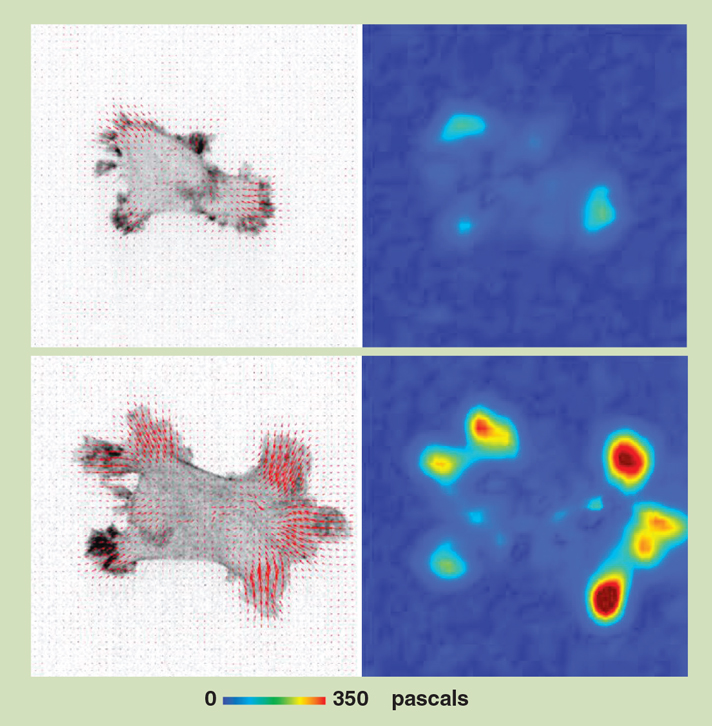
A vector map (above, left column) shows a cell’s direction of movement (red marks) and a force map (right column) shows how much a cell is pressing down on a surface that it is sitting on. The top row shows the cell in a low-viscosity fluid, and the cell is exerting very little pressure. The bottom row shows how the cell quickly spreads out and its pressure increases when it is immersed in a high-viscosity fluid, more like conditions within the body.
Images from Pittman, M., et al., Nature Physics 18:1112.
Controlling the viscous environment of cells could eventually have medical implications. Making an area more viscous could, Chen says, promote wound healing. On the other hand, reducing viscosity might slow down other diseases. For example, fibroblasts, found in connective tissue, cause scarring as well as healing. Overactive fibroblasts are behind the lung disease cystic fibrosis, so conditions that make fibroblasts move faster could exacerbate the disease. “Researchers know that mucus buildup is bad because it makes it hard for patients to breathe, but people have never thought about how this could make things worse and worse,” Chen says. In addition, she notes, some cancer cells are known to secrete substances that make mucus thicker, and aiding the cancer cells’ ability to spread might be why they do it.
Although the biomolecular workings inside a cell are behind what makes the cell move differently, Chen points out that the team’s findings were based on imaging: “We use every pixel available to tell us something quantitative. We measured the area of the cells at specific time points, and we can count each focal adhesion and how long it lasts. Images are not just something beautiful to look at; they have important hidden messages. But if you can dig them out, they’re as powerful as biochemistry or molecular biology.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.