
This Article From Issue
January-February 1999
Volume 87, Number 1
Page 21
DOI: 10.1511/1999.16.21
Chemistry is A + B → C + D, substances and their transformations. Change, symbolized by the arrow in the equation, was always of real value to people, whether it was in the protochemistries of food preparation, gunpowder, dyes or metallurgy. The hold was psychological as well as economic; it was fascinating, frightening, thrilling—all of these—to see color changes, healing and explosions. As chemistry became a molecular science, the magic of transformation did not abate. And the value of adding value to the natural by human transformation never left us, it only changed scale; aside from airplanes and computers, the chemical industry is pretty much the major positive contributor to the U.S. trade balance.
But all along it has been too easy to focus on the before and after, on the reactants and products, on the noun and not the verb. Nor did much change as we learned of the molecules within macroscopic matter—to capture change itself, to visualize it and harness its special features was never easy. The blinking of an eye is glacially slow compared with what happens in the first few of dozens of reactions in photosynthesis, or in the controlled explosions in your car engine.
There is a change in chemistry today, for the arrow is now well on its way to being understood. (See "Reaction Dynamics in Organic Chemistry," March–April 1997.) We have femtosecond spectroscopies that allow us to freeze the arrow's motion in neo-Zeno ways, as in the remarkable work of Ahmed Zewail of Caltech. The pictures we obtain of molecular motion, albeit indirect, are truly as startling, and as decisive of argument, as the photographs that Eadweard Muybridge obtained of galloping horses in the late 19th century (a point made to me by my Stanford colleague John Brauman).
And, in the true test of understanding, we can bend the arrow, direct the reaction elsewhere. Why should we wish to do so? Because we desire that which is not easily given unto us. Or we may profit from this capability. But mainly we wish to gain confidence in our understanding of how transformation takes place by affecting the transformation, by ringing the changes on change itself. In the first installment of Marginalia on the new kinetic art, I tell here two contemporary stories of reaction dynamics.
Chemistry with a Hammer
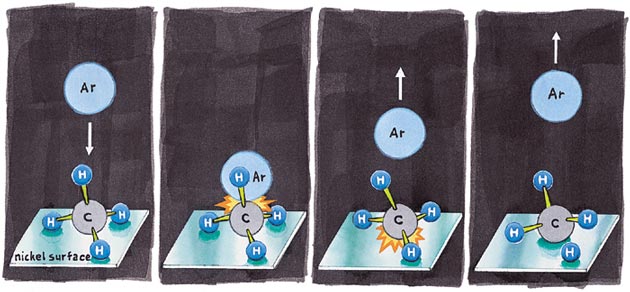
Methane, CH4, "wants" to fall apart or dissociate on a nickel surface to produce CH3 and H strongly bound (chemisorbed) to that surface—the process is thermodynamically downhill. But bleed a little CH4 into the vacuum above a clean nickel surface and nothing happens. Oh, the methane settles down on the surface (physisorption) but refuses to decompose. We say there is a barrier to dissociative chemisorption. Breaking up is hard to do; that we know. But breaking up methane is crucial; if one wants to make complex molecules from abundant natural gas, one better begin by disrupting at least one C–H bond in ever-so-stable methane.
Tom Dunne; Image adapted from a drawing by Sylvia Ceyer, MIT.
The nature and size of that barrier was studied independently by three groups (Gert Ehrlich at the University of Illinois, Robert J. Madix at Stanford and Sylvia T. Ceyer at MIT). They seeded CH4 in a helium beam in a way such that the speed of the CH4 could be controlled. When that beam of CH4 molecules impinged on the surface, nothing happened until the kinetic energy of the methane exceeded a threshold value (about 60 kilojoules per mole), at which point the CH4 fell apart, generating the products CH3 and H, both of which were chemisorbed on the surface.
Sylvia Ceyer and her coworkers then went on to do a beautifully simple experiment. They laid down a layer of gently physisorbed (and thus essentially unperturbed) methane on that surface. Then they trained a beam of argon atoms (the hammer!) of controlled energy on the unsuspecting quiescent methane. When the argon atoms provided somewhat more collisional oomph than the moving methane molecules had in the absence of the noble gas, chemistry took place.
The Quantum World
Though mysteries remain aplenty in the falling apart of molecules at surfaces, the beautiful story I just told was pretty classical. Sometimes the dynamics of chemical reactions have truly nonclassical features, ones that derive from the quantum nature of molecules. For molecules are truly betwixt and between—part classical objects, sculpted in their multifarious richness as macroscopic structures are, part quantum objects with the seemingly arcane consequences of the quantum world.
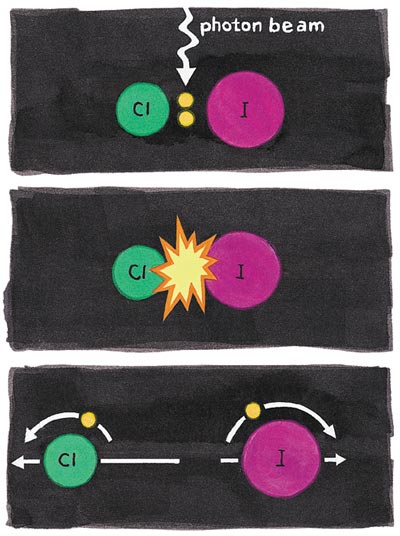
A remarkable report recently appeared from Richard Zare's Stanford University group that exemplifies quantum mysteries, even as it has a partially classical explanation. Diatomic molecules can be induced to fall apart by either heat or light. The Stanford group looked at a simple diatomic, ICl (the mixed partner to familiar diatomic chlorine, Cl2, and iodine, I2) and its photodissociation by a beam of linearly polarized green light. Off come Cl and I atom fragments; the Cl atom is the one they studied.
Tom Dunne; Image adapted from a drawing by Andrew Alexander and Richard Zare, Stanford.
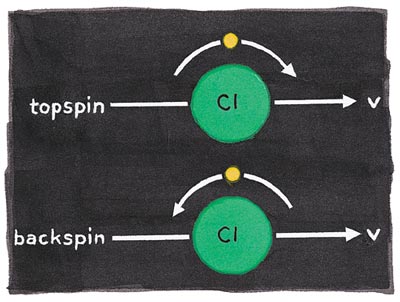
The electron in the Cl atom has two kinds of angular momentum—one is its intrinsic spin, the other the angular momentum of its orbital motion. The two kinds of Cl atoms in which (as the Cl sails away) the odd electron is rotating clockwise or counterclockwise (thus with "topspin" or "backspin" with respect to the direction of motion) can be experimentally distinguished.
The remarkable outcome of the quantum nature of the bond-breaking process is that this "handedness" (helicity) of the electronic angular momentum oscillates between backspin and topspin, depending on the wavelength (thus energy) of the dissociating light! The explanation comes only from the wavelike properties of the material molecule, more precisely from the interference of wave-like descriptions of the mixture of different electronic states accessed by the absorption of that light.
Tom Dunne; Image adapted from a drawing by Andrew Alexander and Richard Zare, Stanford.
Actually, a classical model gets us part of the way to understanding what transpires. The absorption of light starts the electron cloud in the ICl molecule oscillating. Normally, such oscillations have the same symmetry as the molecule; that is, the oscillation occurs either along the bond or at right angles to the bond. In the particular case that Zare's group studies, the excitation of the electron cloud is to a mixed state in which the electron cloud oscillates both along the bond axis and at right angles to it because of quantum-interference effects. The resulting electron motion is a circulation (the helicity) whose sense of rotation depends on how the two different motions are combined. The fragments carry away in the topspin or backspin the memory of that interference.
Beam Me Down
Both of the studies described above depend on supersonic molecular beams expanding into a vacuum—in Ceyer's case a beam of neon, argon or krypton seeded in helium, in Zare's case a pulsed beam source of ICl molecules. The velocities of the molecules emerging from these beams can be controlled by heating the expansion nozzle over a wide temperature range. The beams are "skimmed" downstream by sharp-edged openings and collimated by slits, obstructions and otherwise-shaped orifices. The language of handling tenuous gases is one of machined metals—walking down the lanes of exhibitor's wares at a meeting of the American Vacuum Society is a fantastic experience (in the advertisers' wares, I once found a poem). The language is one of intended control. The outcome, if everything is just right (days of alignment and pumping to get it there), is a molecular beam exquisitely well defined in its dimensions and in the speed of the molecules coursing down their appointed paths.
The Arrow in a New Light
The light is that of lasers, in the service of transforming change itself. Chemists have always been interested in mechanism, the answer to the simple question "How? How does it happen?" The response comes out of looking closer at the arrow in A + B → C + D, finding the many sequential arrows hidden in the gross transformation. The newer work, both femtosecond spectroscopy (to be discussed in a future Marginalia) and the two studies mentioned here, consistently uses lasers. They deliver precise dollops of energy, to disrupt or characterize bonds and motions in a molecule. In the laser's bright light, we are seeing signposts, I think, to a new dynamics.
© Roald Hoffmann

American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.